Giulia Vadori
CISCA and CytoDArk0: a Cell Instance Segmentation and Classification method for histo(patho)logical image Analyses and a new, open, Nissl-stained dataset for brain cytoarchitecture studies
Sep 06, 2024Abstract:Delineating and classifying individual cells in microscopy tissue images is a complex task, yet it is a pivotal endeavor in various medical and biological investigations. We propose a new deep learning framework (CISCA) for automatic cell instance segmentation and classification in histological slices to support detailed morphological and structural analysis or straightforward cell counting in digital pathology workflows and brain cytoarchitecture studies. At the core of CISCA lies a network architecture featuring a lightweight U-Net with three heads in the decoder. The first head classifies pixels into boundaries between neighboring cells, cell bodies, and background, while the second head regresses four distance maps along four directions. The network outputs from the first and second heads are integrated through a tailored post-processing step, which ultimately yields the segmentation of individual cells. A third head enables simultaneous classification of cells into relevant classes, if required. We showcase the effectiveness of our method using four datasets, including CoNIC, PanNuke, and MoNuSeg, which are publicly available H\&E datasets. Additionally, we introduce CytoDArk0, a novel dataset consisting of Nissl-stained images of the cortex, cerebellum, and hippocampus from mammals belonging to the orders Cetartiodactyla and Primates. We evaluate CISCA in comparison to other state-of-the-art methods, demonstrating CISCA's robustness and accuracy in segmenting and classifying cells across diverse tissue types, magnifications, and staining techniques.
Revealing Cortical Layers In Histological Brain Images With Self-Supervised Graph Convolutional Networks Applied To Cell-Graphs
Nov 26, 2023
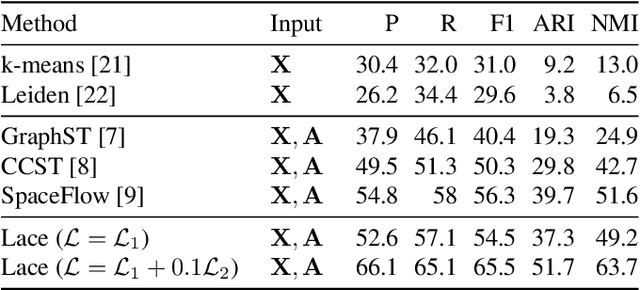
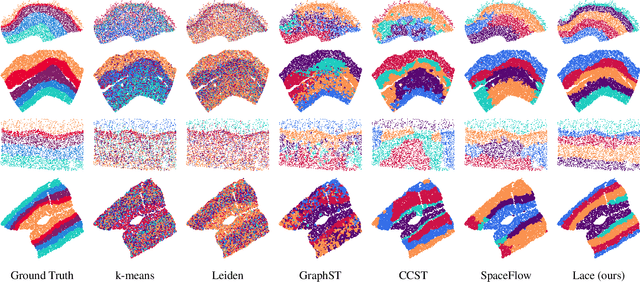
Abstract:Identifying cerebral cortex layers is crucial for comparative studies of the cytoarchitecture aiming at providing insights into the relations between brain structure and function across species. The absence of extensive annotated datasets typically limits the adoption of machine learning approaches, leading to the manual delineation of cortical layers by neuroanatomists. We introduce a self-supervised approach to detect layers in 2D Nissl-stained histological slices of the cerebral cortex. It starts with the segmentation of individual cells and the creation of an attributed cell-graph. A self-supervised graph convolutional network generates cell embeddings that encode morphological and structural traits of the cellular environment and are exploited by a community detection algorithm for the final layering. Our method, the first self-supervised of its kind with no spatial transcriptomics data involved, holds the potential to accelerate cytoarchitecture analyses, sidestepping annotation needs and advancing cross-species investigation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge