Fabian Coupette
Bayesian unsupervised learning reveals hidden structure in concentrated electrolytes
Dec 19, 2020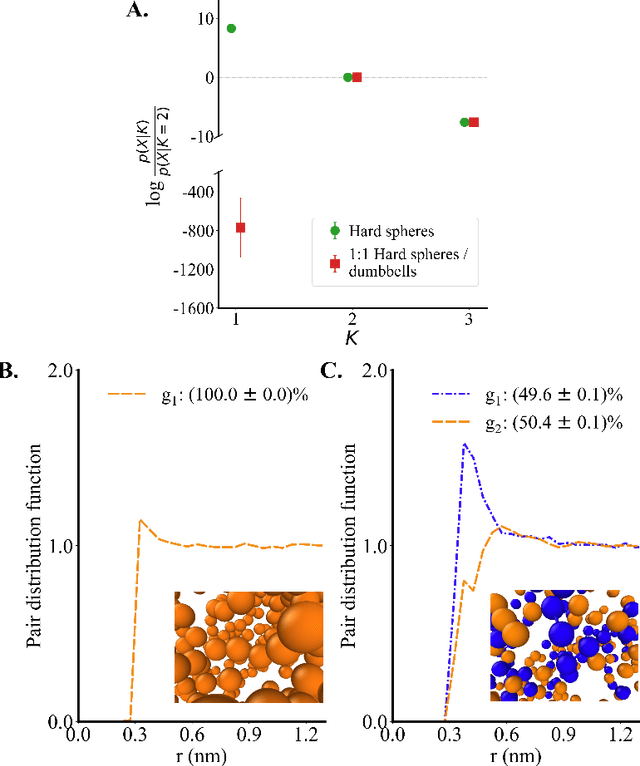
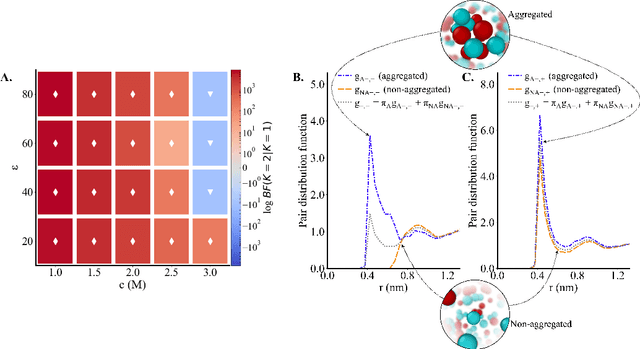
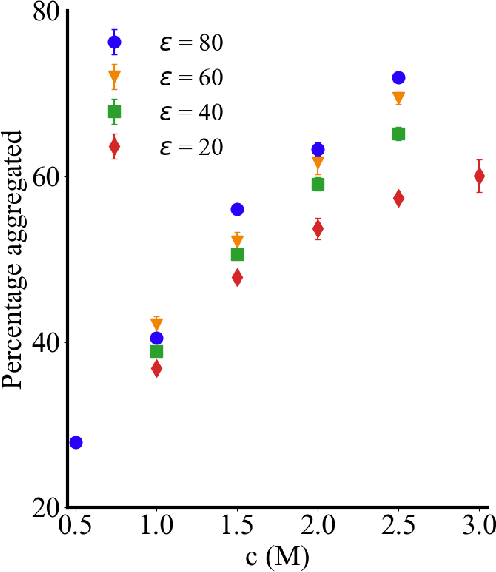
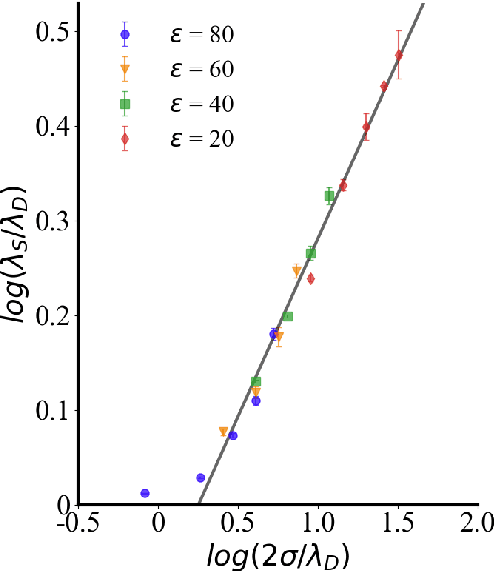
Abstract:Electrolytes play an important role in a plethora of applications ranging from energy storage to biomaterials. Notwithstanding this, the structure of concentrated electrolytes remains enigmatic. Many theoretical approaches attempt to model the concentrated electrolytes by introducing the idea of ion pairs, with ions either being tightly `paired' with a counter-ion, or `free' to screen charge. In this study we reframe the problem into the language of computational statistics, and test the null hypothesis that all ions share the same local environment. Applying the framework to molecular dynamics simulations, we show that this null hypothesis is not supported by data. Our statistical technique suggests the presence of distinct local ionic environments; surprisingly, these differences arise in like charge correlations rather than unlike charge attraction. The resulting fraction of particles in non-aggregated environments shows a universal scaling behaviour across different background dielectric constants and ionic concentrations.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge