Dominique Duncan
Robust and Interpretable COVID-19 Diagnosis on Chest X-ray Images using Adversarial Training
Nov 23, 2023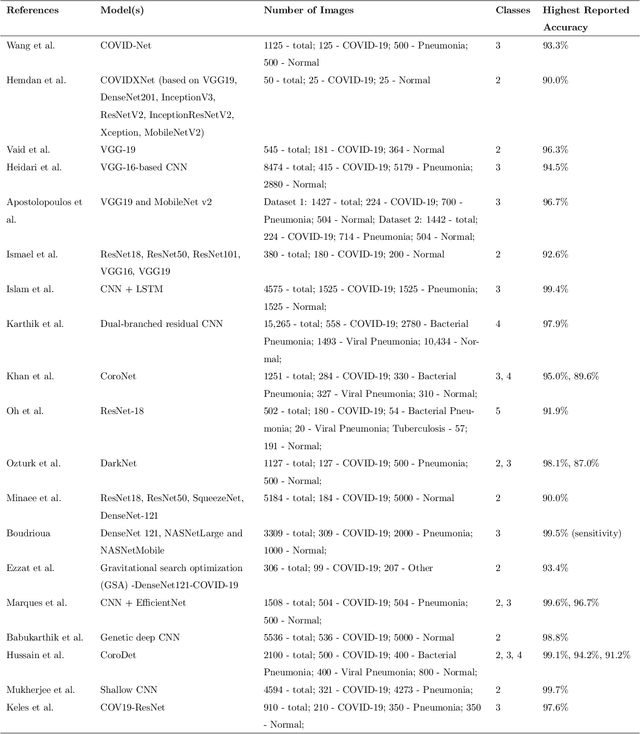

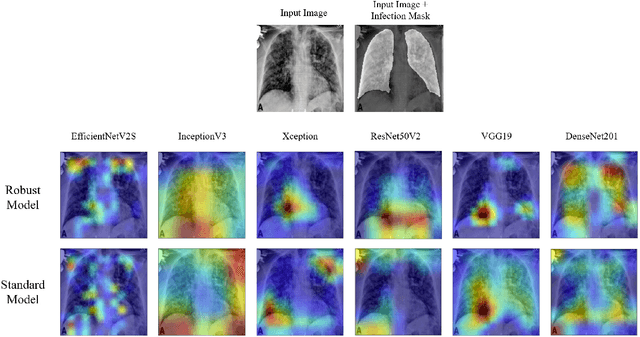
Abstract:The novel 2019 Coronavirus disease (COVID-19) global pandemic is a defining health crisis. Recent efforts have been increasingly directed towards achieving quick and accurate detection of COVID-19 across symptomatic patients to mitigate the intensity and spread of the disease. Artificial intelligence (AI) algorithms applied to chest X-ray (CXR) images have emerged as promising diagnostic tools, and previous work has demonstrated impressive classification performances. However, such methods have faced criticisms from physicians due to their black-box reasoning process and unpredictable nature. In contrast to professional radiologist diagnosis, AI systems often lack generalizability, explainability, and robustness in the clinical decision making process. In our work, we address these issues by first proposing an extensive baseline study, training and evaluating 21 convolutional neural network (CNN) models on a diverse set of 33,000+ CXR images to classify between healthy, COVID-19, and non-COVID-19 pneumonia CXRs. Our resulting models achieved a 3-way classification accuracy, recall, and precision of up to 97.03\%, 97.97\%, and 99.95\%, respectively. Next, we investigate the effectiveness of adversarial training on model robustness and explainability via Gradient-weighted Class Activation Mapping (Grad-CAM) heatmaps. We find that adversarially trained models not only significantly outperform their standard counterparts on classifying perturbed images, but also yield saliency maps that 1) better specify clinically relevant features, 2) are robust against extraneous artifacts, and 3) agree considerably more with expert radiologist findings.
Unsupervised Multivariate Time-Series Transformers for Seizure Identification on EEG
Jan 03, 2023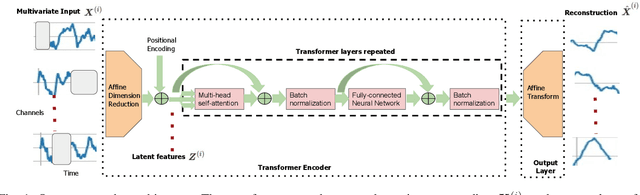
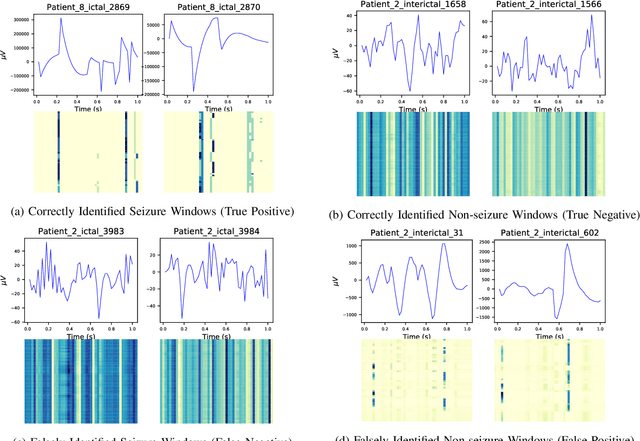
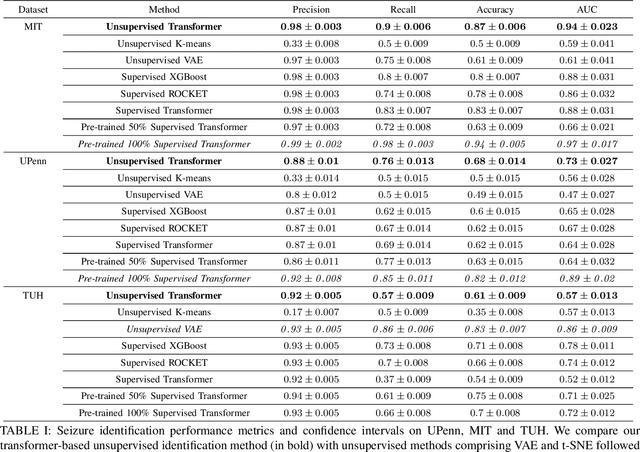
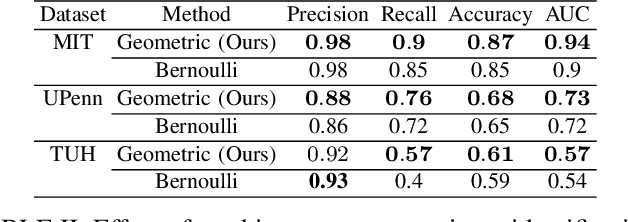
Abstract:Epilepsy is one of the most common neurological disorders, typically observed via seizure episodes. Epileptic seizures are commonly monitored through electroencephalogram (EEG) recordings due to their routine and low expense collection. The stochastic nature of EEG makes seizure identification via manual inspections performed by highly-trained experts a tedious endeavor, motivating the use of automated identification. The literature on automated identification focuses mostly on supervised learning methods requiring expert labels of EEG segments that contain seizures, which are difficult to obtain. Motivated by these observations, we pose seizure identification as an unsupervised anomaly detection problem. To this end, we employ the first unsupervised transformer-based model for seizure identification on raw EEG. We train an autoencoder involving a transformer encoder via an unsupervised loss function, incorporating a novel masking strategy uniquely designed for multivariate time-series data such as EEG. Training employs EEG recordings that do not contain any seizures, while seizures are identified with respect to reconstruction errors at inference time. We evaluate our method on three publicly available benchmark EEG datasets for distinguishing seizure vs. non-seizure windows. Our method leads to significantly better seizure identification performance than supervised learning counterparts, by up to 16% recall, 9% accuracy, and 9% Area under the Receiver Operating Characteristics Curve (AUC), establishing particular benefits on highly imbalanced data. Through accurate seizure identification, our method could facilitate widely accessible and early detection of epilepsy development, without needing expensive label collection or manual feature extraction.
Efficient and Visualizable Convolutional Neural Networks for COVID-19 Classification Using Chest CT
Dec 22, 2020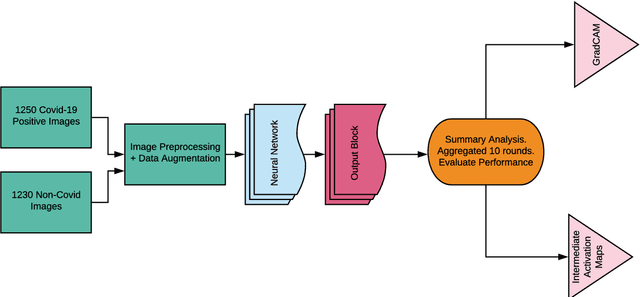
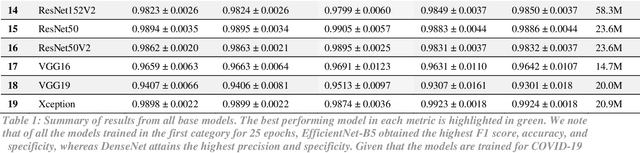
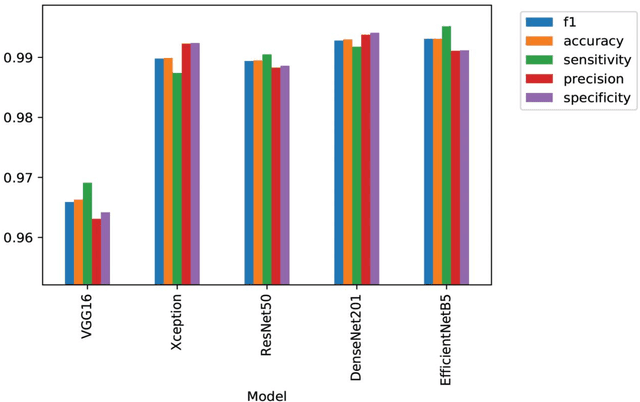
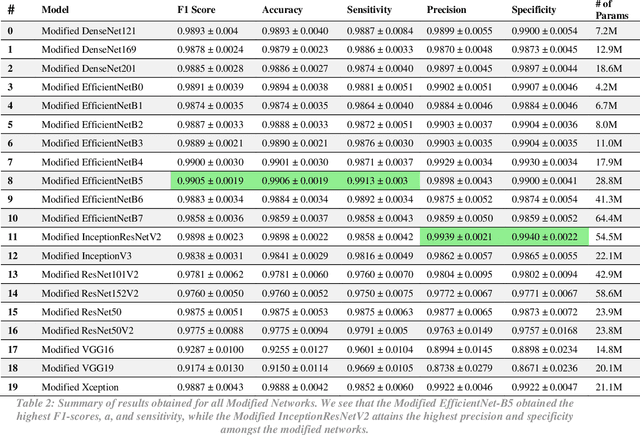
Abstract:The novel 2019 coronavirus disease (COVID-19) has infected over 65 million people worldwide as of December 4, 2020, pushing the world to the brink of social and economic collapse. With cases rising rapidly, deep learning has emerged as a promising diagnosis technique. However, identifying the most accurate models to characterize COVID-19 patients is challenging because comparing results obtained with different types of data and acquisition processes is non-trivial. In this paper, we evaluated and compared 40 different convolutional neural network architectures for COVID-19 diagnosis, serving as the first to consider the EfficientNet family for COVID-19 diagnosis. EfficientNet-B5 is identified as the best model with an accuracy of 0.9931+/-0.0021, F1 score of 0.9931+/-0.0020, sensitivity of 0.9952+/-0.0020, and specificity of 0.9912+/-0.0048. Intermediate activation maps and Gradient-weighted Class Activation Mappings offer human-interpretable evidence of the model's perception of ground-class opacities and consolidations, hinting towards a promising use-case of artificial intelligence-assisted radiology tools.
Prediction of Epilepsy Development in Traumatic Brain Injury Patients from Diffusion Weighted MRI
May 01, 2020

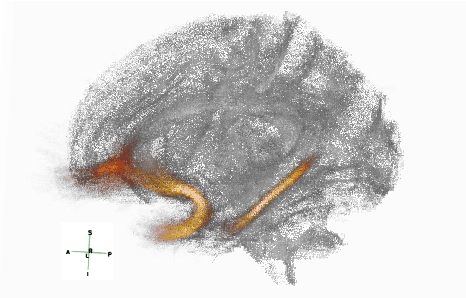
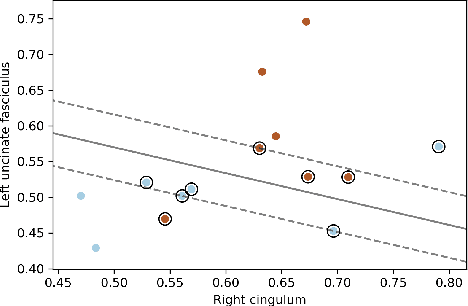
Abstract:Post-traumatic epilepsy (PTE) is a life-long complication of traumatic brain injury (TBI) and is a major public health problem that has an estimated incidence that ranges from 2%-50%, depending on the severity of the TBI. Currently, the pathomechanism that in-duces epileptogenesis in TBI patients is unclear, and one of the most challenging goals in the epilepsy community is to predict which TBI patients will develop epilepsy. In this work, we used diffusion-weighted imaging (DWI) of 14 TBI patients recruited in the Epilepsy Bioinformatics Study for Antiepileptogenic Therapy (EpiBioS4Rx)to measure and analyze fractional anisotropy (FA), obtained from tract-based spatial statistic (TBSS) analysis. Then we used these measurements to train two support vector machine (SVM) models to predict which TBI patients have developed epilepsy. Our approach, tested on these 14 patients with a leave-two-out cross-validation, allowed us to obtain an accuracy of 0.857 $\pm$ 0.18 (with a 95% level of confidence), demonstrating it to be potentially promising for the early characterization of PTE.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge