Denis Constales
Data Driven Reaction Mechanism Estimation via Transient Kinetics and Machine Learning
Nov 17, 2020
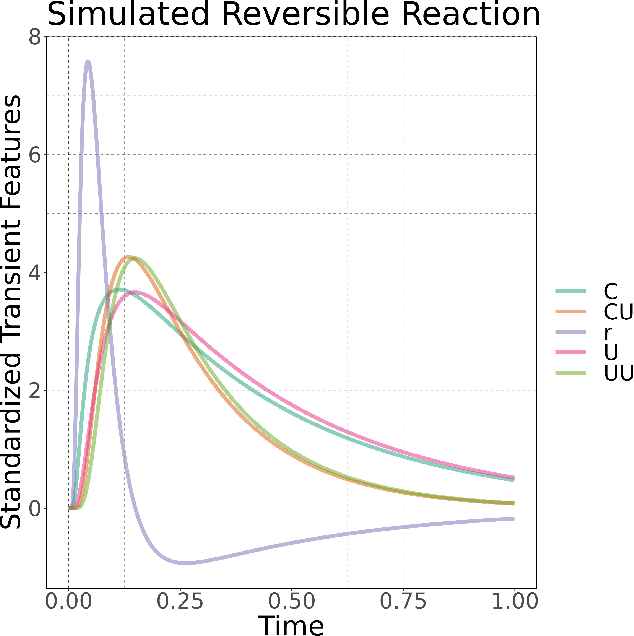

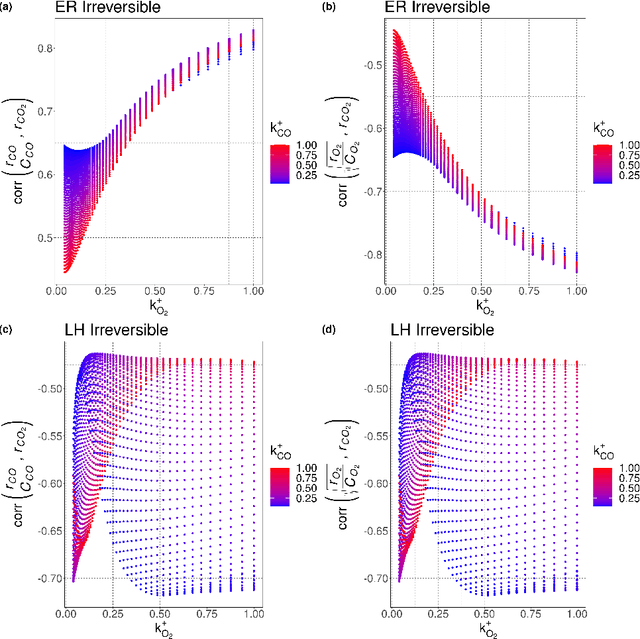
Abstract:Understanding the set of elementary steps and kinetics in each reaction is extremely valuable to make informed decisions about creating the next generation of catalytic materials. With physical and mechanistic complexity of industrial catalysts, it is critical to obtain kinetic information through experimental methods. As such, this work details a methodology based on the combination of transient rate/concentration dependencies and machine learning to measure the number of active sites, the individual rate constants, and gain insight into the mechanism under a complex set of elementary steps. This new methodology was applied to simulated transient responses to verify its ability to obtain correct estimates of the micro-kinetic coefficients. Furthermore, experimental CO oxidation data was analyzed to reveal the Langmuir-Hinshelwood mechanism driving the reaction. As oxygen accumulated on the catalyst, a transition in the mechanism was clearly defined in the machine learning analysis due to the large amount of kinetic information available from transient reaction techniques. This methodology is proposed as a new data driven approach to characterize how materials control complex reaction mechanisms relying exclusively on experimental data.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge