Daria Frolova
Redesigning Out-of-Distribution Detection on 3D Medical Images
Aug 07, 2023Abstract:Detecting out-of-distribution (OOD) samples for trusted medical image segmentation remains a significant challenge. The critical issue here is the lack of a strict definition of abnormal data, which often results in artificial problem settings without measurable clinical impact. In this paper, we redesign the OOD detection problem according to the specifics of volumetric medical imaging and related downstream tasks (e.g., segmentation). We propose using the downstream model's performance as a pseudometric between images to define abnormal samples. This approach enables us to weigh different samples based on their performance impact without an explicit ID/OOD distinction. We incorporate this weighting in a new metric called Expected Performance Drop (EPD). EPD is our core contribution to the new problem design, allowing us to rank methods based on their clinical impact. We demonstrate the effectiveness of EPD-based evaluation in 11 CT and MRI OOD detection challenges.
Limitations of Out-of-Distribution Detection in 3D Medical Image Segmentation
Jun 23, 2023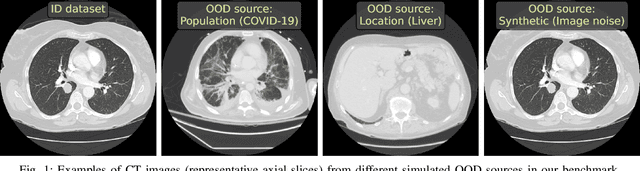
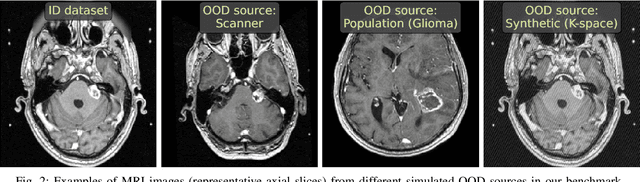
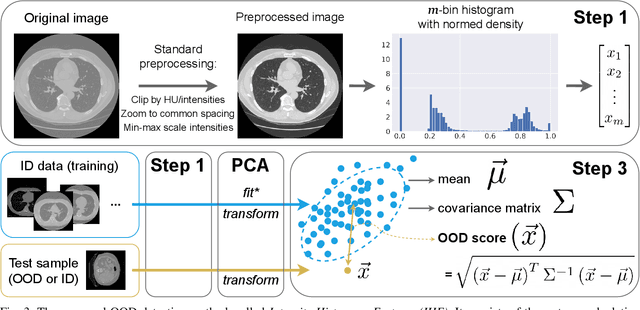
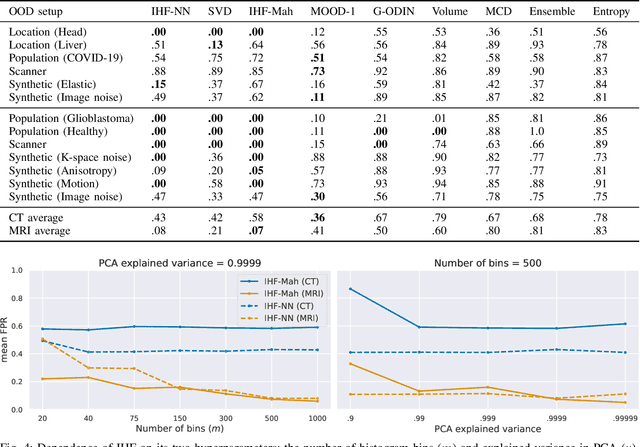
Abstract:Deep Learning models perform unreliably when the data comes from a distribution different from the training one. In critical applications such as medical imaging, out-of-distribution (OOD) detection methods help to identify such data samples, preventing erroneous predictions. In this paper, we further investigate the OOD detection effectiveness when applied to 3D medical image segmentation. We design several OOD challenges representing clinically occurring cases and show that none of these methods achieve acceptable performance. Methods not dedicated to segmentation severely fail to perform in the designed setups; their best mean false positive rate at 95% true positive rate (FPR) is 0.59. Segmentation-dedicated ones still achieve suboptimal performance, with the best mean FPR of 0.31 (lower is better). To indicate this suboptimality, we develop a simple method called Intensity Histogram Features (IHF), which performs comparable or better in the same challenges, with a mean FPR of 0.25. Our findings highlight the limitations of the existing OOD detection methods on 3D medical images and present a promising avenue for improving them. To facilitate research in this area, we release the designed challenges as a publicly available benchmark and formulate practical criteria to test the OOD detection generalization beyond the suggested benchmark. We also propose IHF as a solid baseline to contest the emerging methods.
Solving Sample-Level Out-of-Distribution Detection on 3D Medical Images
Dec 13, 2022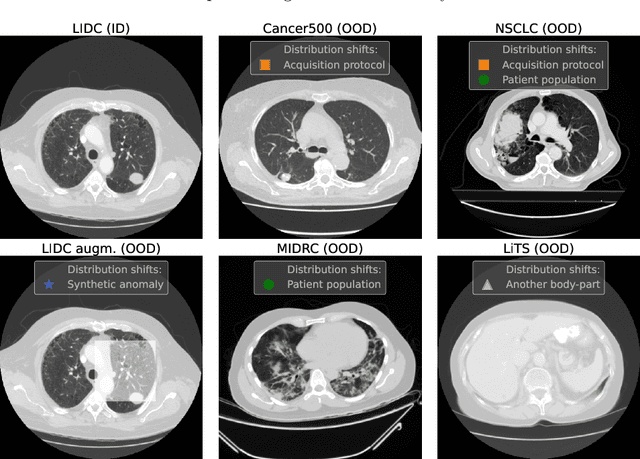
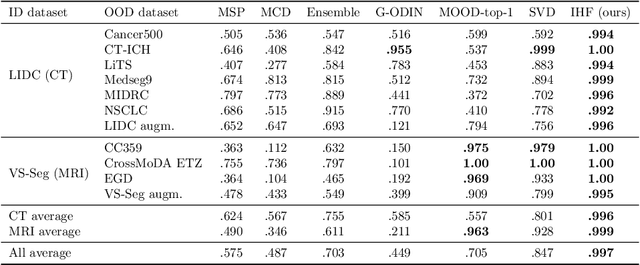
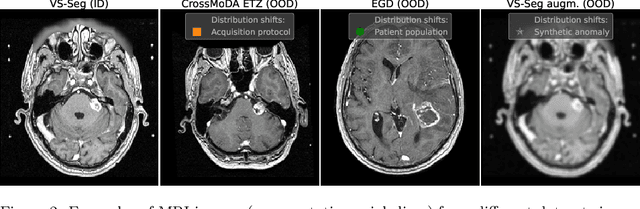
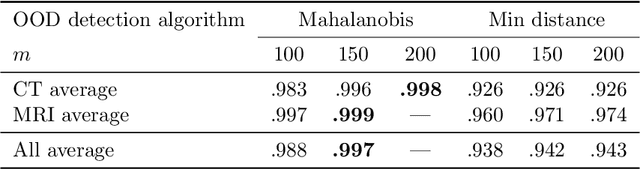
Abstract:Deep Learning (DL) models tend to perform poorly when the data comes from a distribution different from the training one. In critical applications such as medical imaging, out-of-distribution (OOD) detection helps to identify such data samples, increasing the model's reliability. Recent works have developed DL-based OOD detection that achieves promising results on 2D medical images. However, scaling most of these approaches on 3D images is computationally intractable. Furthermore, the current 3D solutions struggle to achieve acceptable results in detecting even synthetic OOD samples. Such limited performance might indicate that DL often inefficiently embeds large volumetric images. We argue that using the intensity histogram of the original CT or MRI scan as embedding is descriptive enough to run OOD detection. Therefore, we propose a histogram-based method that requires no DL and achieves almost perfect results in this domain. Our proposal is supported two-fold. We evaluate the performance on the publicly available datasets, where our method scores 1.0 AUROC in most setups. And we score second in the Medical Out-of-Distribution challenge without fine-tuning and exploiting task-specific knowledge. Carefully discussing the limitations, we conclude that our method solves the sample-level OOD detection on 3D medical images in the current setting.
Exploring Structure-Wise Uncertainty for 3D Medical Image Segmentation
Nov 01, 2022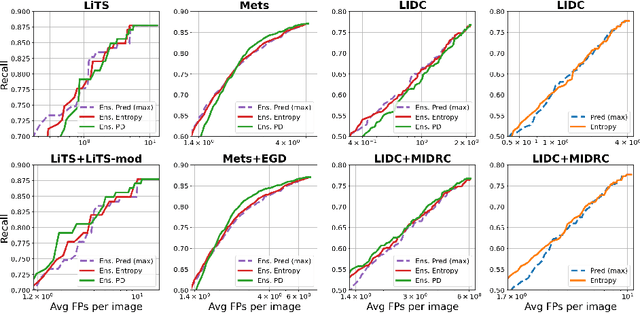
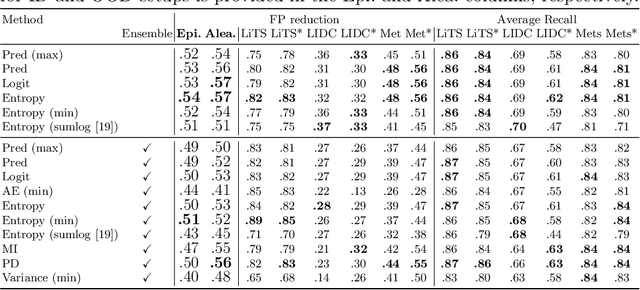
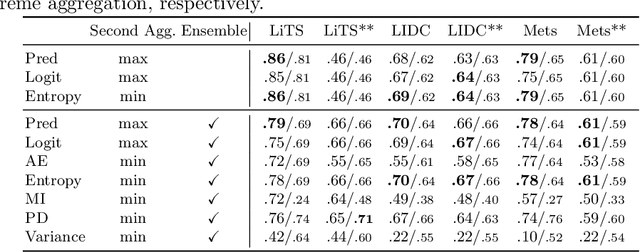
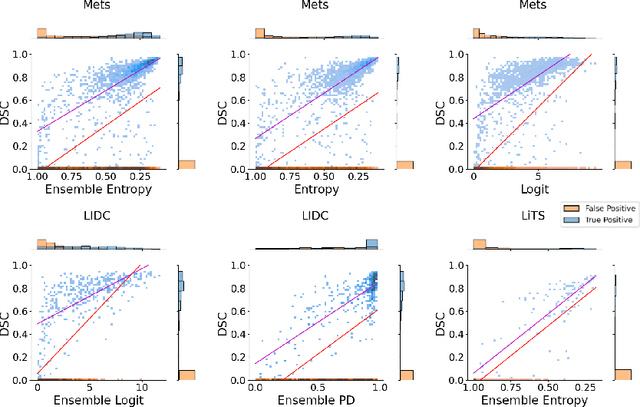
Abstract:When applying a Deep Learning model to medical images, it is crucial to estimate the model uncertainty. Voxel-wise uncertainty is a useful visual marker for human experts and could be used to improve the model's voxel-wise output, such as segmentation. Moreover, uncertainty provides a solid foundation for out-of-distribution (OOD) detection, improving the model performance on the image-wise level. However, one of the frequent tasks in medical imaging is the segmentation of distinct, local structures such as tumors or lesions. Here, the structure-wise uncertainty allows more precise operations than image-wise and more semantic-aware than voxel-wise. The way to produce uncertainty for individual structures remains poorly explored. We propose a framework to measure the structure-wise uncertainty and evaluate the impact of OOD data on the model performance. Thus, we identify the best UE method to improve the segmentation quality. The proposed framework is tested on three datasets with the tumor segmentation task: LIDC-IDRI, LiTS, and a private one with multiple brain metastases cases.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge