Basavaraju G. Sanganahalli
Evaluating U-net Brain Extraction for Multi-site and Longitudinal Preclinical Stroke Imaging
Mar 11, 2022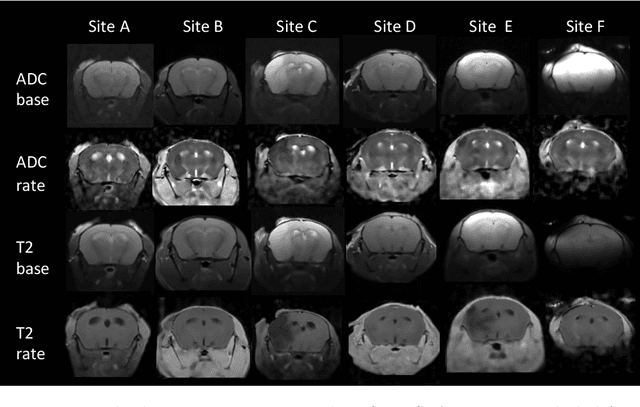
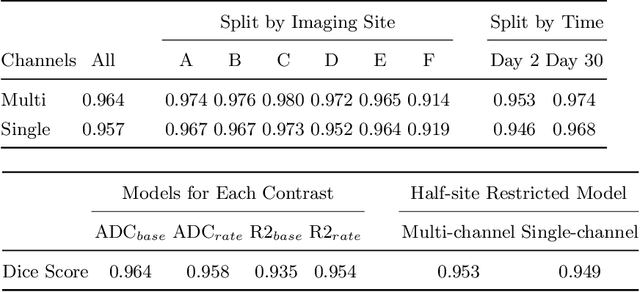
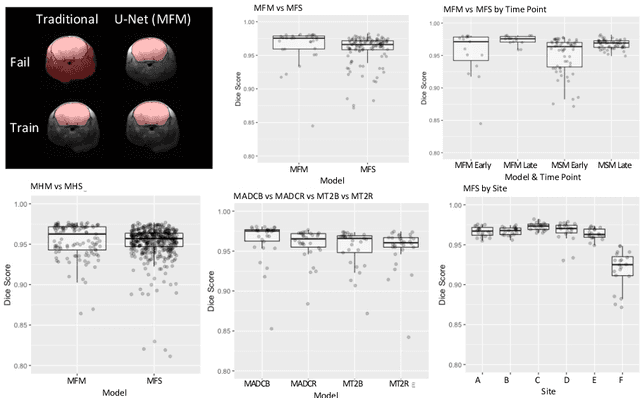
Abstract:Rodent stroke models are important for evaluating treatments and understanding the pathophysiology and behavioral changes of brain ischemia, and magnetic resonance imaging (MRI) is a valuable tool for measuring outcome in preclinical studies. Brain extraction is an essential first step in most neuroimaging pipelines; however, it can be challenging in the presence of severe pathology and when dataset quality is highly variable. Convolutional neural networks (CNNs) can improve accuracy and reduce operator time, facilitating high throughput preclinical studies. As part of an ongoing preclinical stroke imaging study, we developed a deep-learning mouse brain extraction tool by using a U-net CNN. While previous studies have evaluated U-net architectures, we sought to evaluate their practical performance across data types. We ask how performance is affected with data across: six imaging centers, two time points after experimental stroke, and across four MRI contrasts. We trained, validated, and tested a typical U-net model on 240 multimodal MRI datasets including quantitative multi-echo T2 and apparent diffusivity coefficient (ADC) maps, and performed qualitative evaluation with a large preclinical stroke database (N=1,368). We describe the design and development of this system, and report our findings linking data characteristics to segmentation performance. We consistently found high accuracy and ability of the U-net architecture to generalize performance in a range of 95-97% accuracy, with only modest reductions in performance based on lower fidelity imaging hardware and brain pathology. This work can help inform the design of future preclinical rodent imaging studies and improve their scalability and reliability.
Image-based Stroke Assessment for Multi-site Preclinical Evaluation of Cerebroprotectants
Mar 11, 2022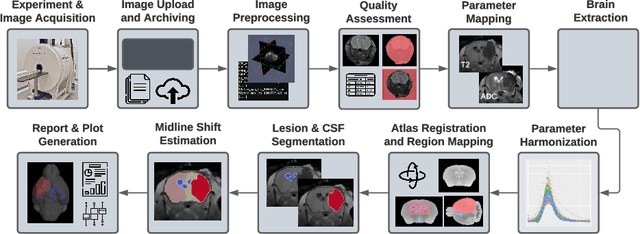
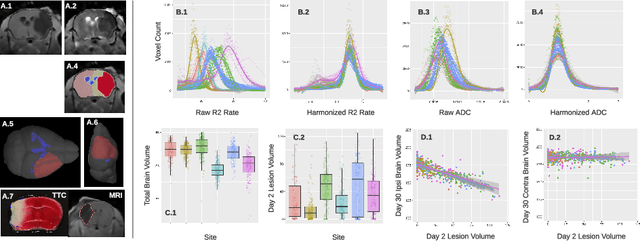
Abstract:Ischemic stroke is a leading cause of death worldwide, but there has been little success translating putative cerebroprotectants from preclinical trials to patients. We investigated computational image-based assessment tools for practical improvement of the quality, scalability, and outlook for large scale preclinical screening for potential therapeutic interventions. We developed, evaluated, and deployed a pipeline for image-based stroke outcome quantification for the Stroke Prelinical Assessment Network (SPAN), which is a multi-site, multi-arm, multi-stage study evaluating a suite of cerebroprotectant interventions. Our fully automated pipeline combines state-of-the-art algorithmic and data analytic approaches to assess stroke outcomes from multi-parameter MRI data collected longitudinally from a rodent model of middle cerebral artery occlusion (MCAO), including measures of infarct volume, brain atrophy, midline shift, and data quality. We tested our approach with 1,368 scans and report population level results of lesion extent and longitudinal changes from injury. We validated our system by comparison with manual annotations of coronal MRI slices and tissue sections from the same brain, using crowdsourcing from blinded stroke experts from the network. Our results demonstrate the efficacy and robustness of our image-based stroke assessments. The pipeline may provide a promising resource for ongoing preclinical studies conducted by SPAN and other networks in the future.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge