Azah Kamilah Muda
Weighted Tanimoto Coefficient for 3D Molecule Structure Similarity Measurement
Jun 10, 2018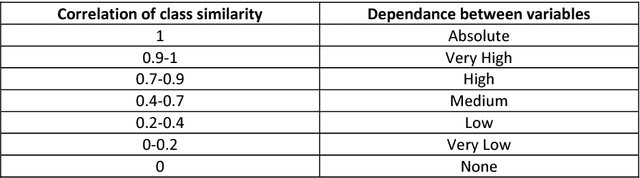
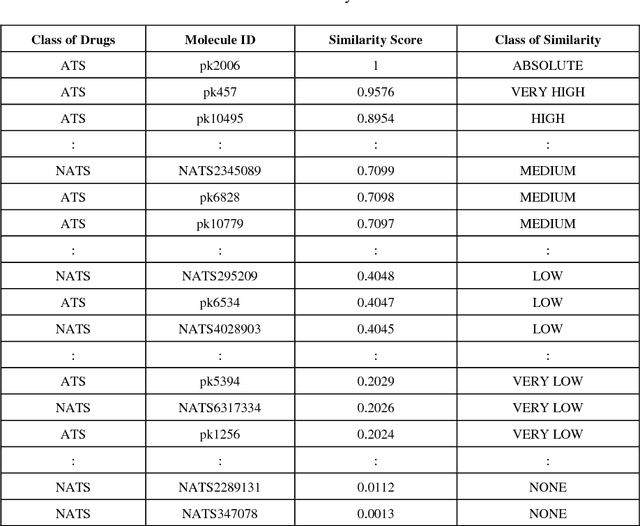
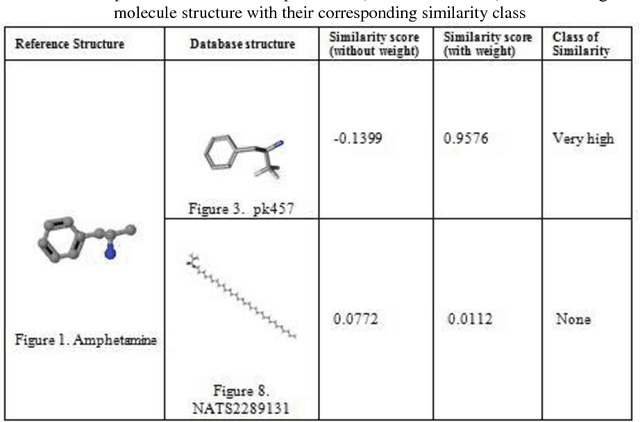
Abstract:Similarity searching of molecular structure has been an important application in the Chemoinformatics, especially in drug discovery. Similarity searching is a common method used for identification of molecular structure. It involve three main principal component of similarity searching: structure representation; weighting scheme; and similarity coefficient. In this paper, we introduces Weighted Tanimoto Coefficient based on weighted Euclidean distance in order to investigate the effect of weight function on the result for similarity searching. The Tanimoto coefficient is one of the popular similarity coefficients used to measure the similarity between pairs of the molecule. The most of research area found that the similarity searching is based on binary or fingerprint data. Meanwhile, we used non-binary data and was set amphetamine structure as a reference or targeted structure and the rest of the dataset becomes a database structure. Throughout this study, it showed that there is definitely gives a different result between a similarity searching with and without weight.
Using 3D Hahn Moments as A Computational Representation of ATS Drugs Molecular Structure
Mar 17, 2018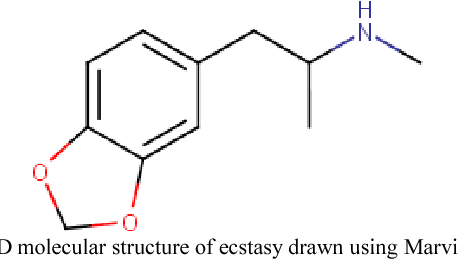
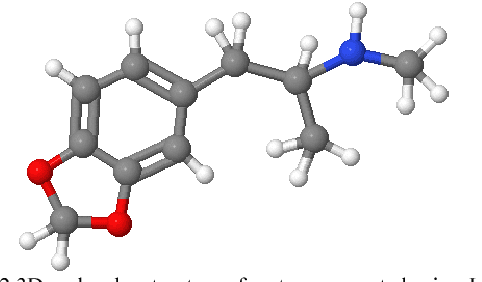
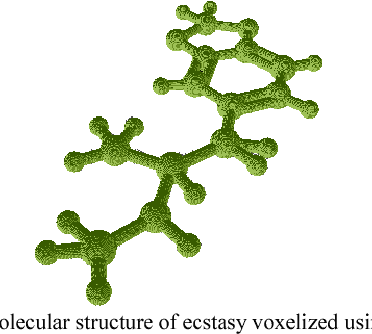
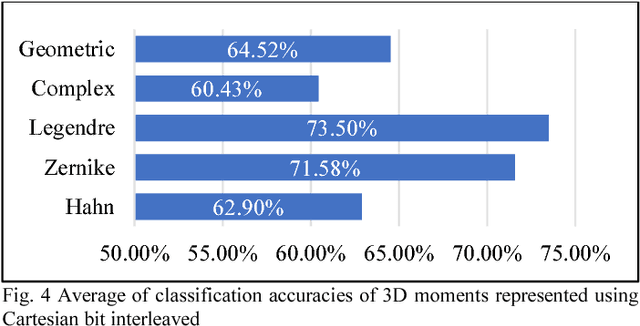
Abstract:The campaign against drug abuse is fought by all countries, most notably on ATS drugs. The technical limitations of the current test kits to detect new brand of ATS drugs present a challenge to law enforcement authorities and forensic laboratories. Meanwhile, new molecular imaging devices which allowed mankind to characterize the physical 3D molecular structure have been recently introduced, and it can be used to remedy the limitations of existing drug test kits. Thus, a new type of 3D molecular structure representation technique should be developed to cater the 3D molecular structure acquired physically using these molecular imaging devices. One of the applications of image processing methods to represent a 3D image is 3D moments, and this study formulates a new 3D moments technique, namely 3D Hahn moments, to represent the 3D molecular structure of ATS drugs. The performance of the proposed technique was analysed using drug chemical structures obtained from UNODC for the ATS drugs, while non-ATS drugs are obtained randomly from ChemSpider database. The evaluation shows the technique is qualified to be further explored in the future works to be fully compatible with ATS drug identification domain.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge