Variational Mixtures of ODEs for Inferring Cellular Gene Expression Dynamics
Paper and Code
Jul 09, 2022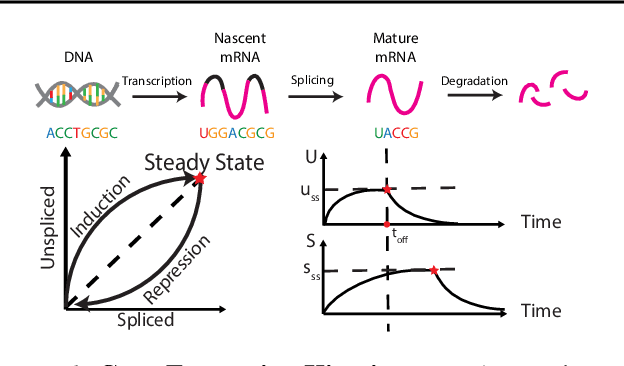
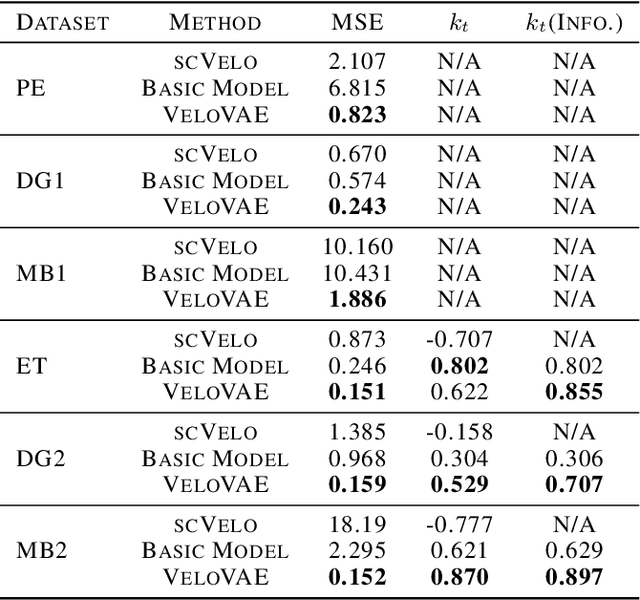
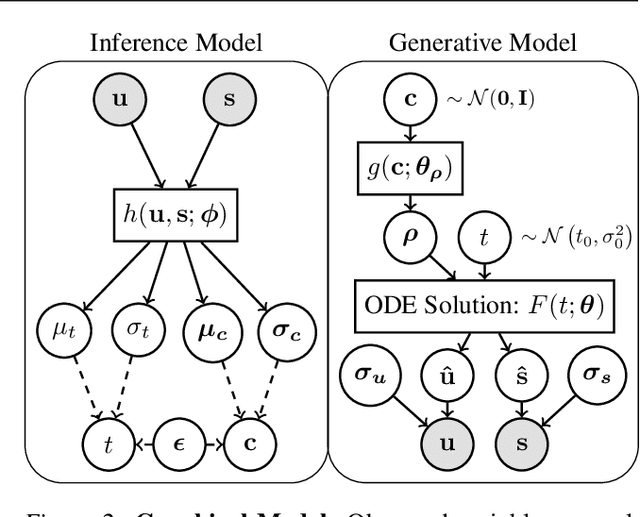
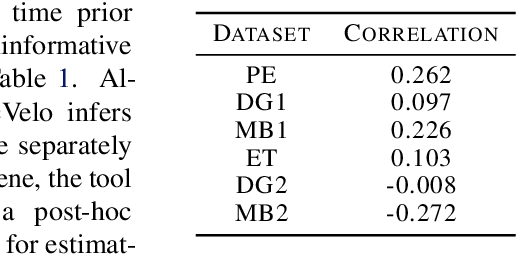
A key problem in computational biology is discovering the gene expression changes that regulate cell fate transitions, in which one cell type turns into another. However, each individual cell cannot be tracked longitudinally, and cells at the same point in real time may be at different stages of the transition process. This can be viewed as a problem of learning the behavior of a dynamical system from observations whose times are unknown. Additionally, a single progenitor cell type often bifurcates into multiple child cell types, further complicating the problem of modeling the dynamics. To address this problem, we developed an approach called variational mixtures of ordinary differential equations. By using a simple family of ODEs informed by the biochemistry of gene expression to constrain the likelihood of a deep generative model, we can simultaneously infer the latent time and latent state of each cell and predict its future gene expression state. The model can be interpreted as a mixture of ODEs whose parameters vary continuously across a latent space of cell states. Our approach dramatically improves data fit, latent time inference, and future cell state estimation of single-cell gene expression data compared to previous approaches.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge