Theory of coupled neuronal-synaptic dynamics
Paper and Code
Feb 17, 2023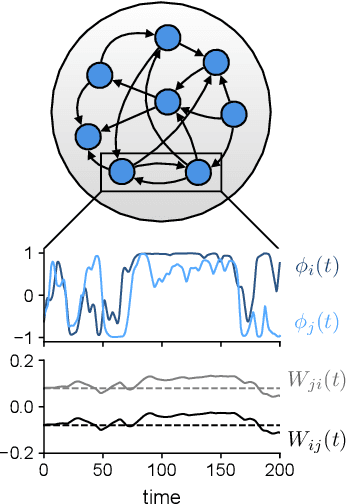
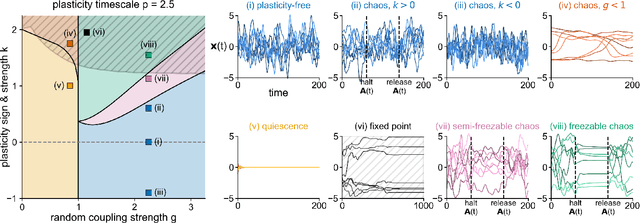
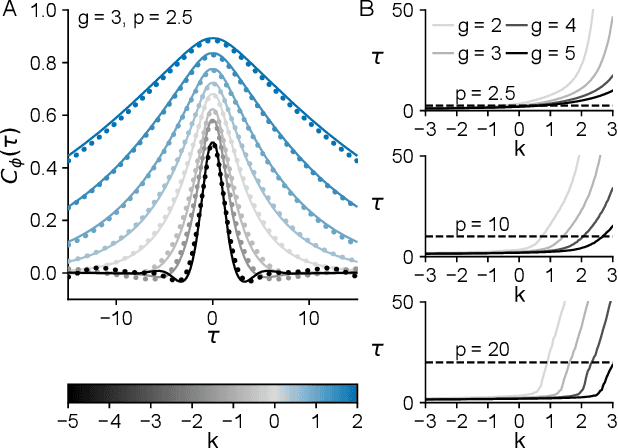
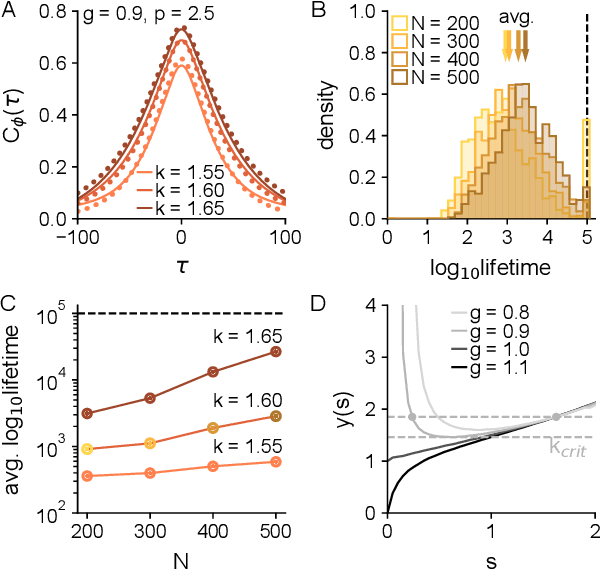
In neural circuits, synapses influence neurons by shaping network dynamics, and neurons influence synapses through activity-dependent plasticity. Motivated by this fact, we study a network model in which neurons and synapses are mutually coupled dynamic variables. Model neurons obey dynamics shaped by synaptic couplings that fluctuate, in turn, about quenched random strengths in response to pre- and postsynaptic neuronal activity. Using dynamical mean-field theory, we compute the phase diagram of the combined neuronal-synaptic system, revealing several novel phases suggestive of computational function. In the regime in which the non-plastic system is chaotic, Hebbian plasticity slows chaos, while anti-Hebbian plasticity quickens chaos and generates an oscillatory component in neuronal activity. Deriving the spectrum of the joint neuronal-synaptic Jacobian reveals that these behaviors manifest as differential effects of eigenvalue repulsion. In the regime in which the non-plastic system is quiescent, Hebbian plasticity can induce chaos. In both regimes, sufficiently strong Hebbian plasticity creates exponentially many stable neuronal-synaptic fixed points that coexist with chaotic states. Finally, in chaotic states with sufficiently strong Hebbian plasticity, halting synaptic dynamics leaves a stable fixed point of neuronal dynamics, freezing the neuronal state. This phase of freezable chaos provides a novel mechanism of synaptic working memory in which a stable fixed point of neuronal dynamics is continuously destabilized through synaptic dynamics, allowing any neuronal state to be stored as a stable fixed point by halting synaptic plasticity.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge