Terahertz Induced Protein Interactions in a Random Medium
Paper and Code
Oct 23, 2023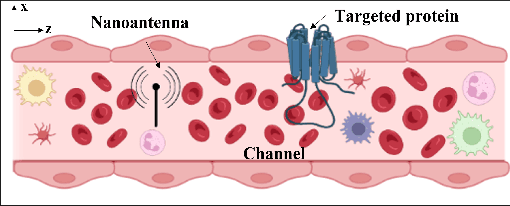
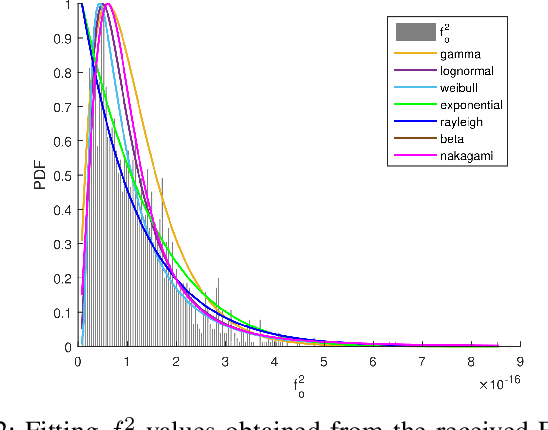
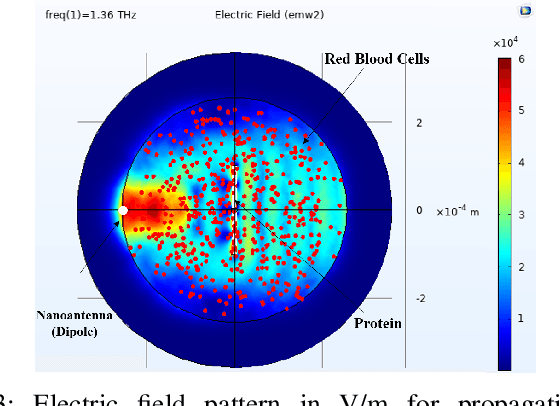
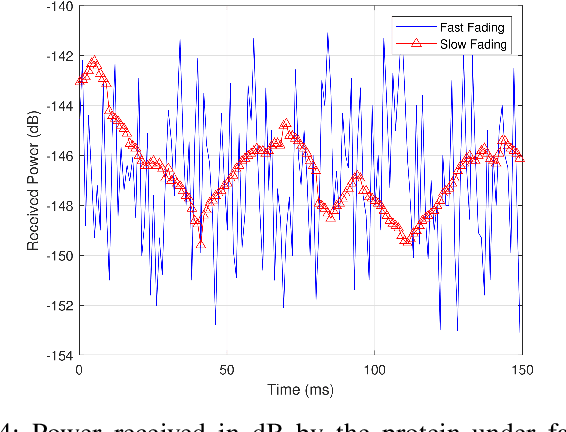
Folding of proteins into their correct native structure is key to their function. Simultaneously, the intricate interplay between cell movement and protein conformation highlights the complex nature of cellular processes. In this work, we demonstrate the impact of Terahertz (THz) signaling on controlling protein conformational changes in a random medium. Our system of interest consists of a communication link that involves a nanoantenna transmitter, a protein receiver, and a channel composed of moving red blood cells. Due to the system dynamics, we investigate the influence of both the fast and slow channel variations on protein folding. Specifically, we analyze the system's selectivity to asses the effectiveness of the induced THz interaction in targeting a specific group of proteins under fading conditions. By optimizing the selectivity metric with respect to the nanoantenna power and frequency, it is possible to enhance the controllability of protein interactions. Our probabilistic analysis provides a new perspective regarding electromagnetically triggered protein molecules, their microenvironment and their interaction with surrounding particles. It helps elucidate how external conditions impact the protein folding kinetics and pathways. This results in not only understanding the mechanisms underlying THz-induced protein interactions but also engineering these still-emerging tools.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge