PhyloTransformer: A Discriminative Model for Mutation Prediction Based on a Multi-head Self-attention Mechanism
Paper and Code
Nov 03, 2021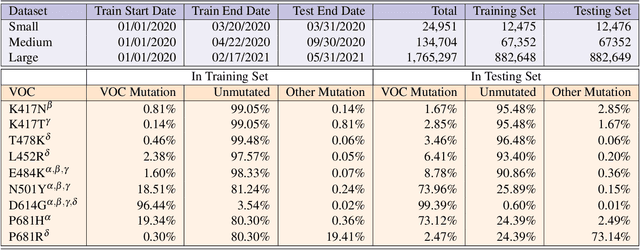
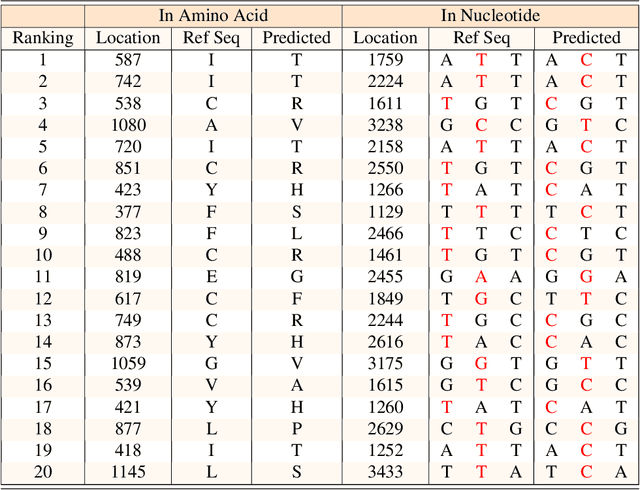
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused an ongoing pandemic infecting 219 million people as of 10/19/21, with a 3.6% mortality rate. Natural selection can generate favorable mutations with improved fitness advantages; however, the identified coronaviruses may be the tip of the iceberg, and potentially more fatal variants of concern (VOCs) may emerge over time. Understanding the patterns of emerging VOCs and forecasting mutations that may lead to gain of function or immune escape is urgently required. Here we developed PhyloTransformer, a Transformer-based discriminative model that engages a multi-head self-attention mechanism to model genetic mutations that may lead to viral reproductive advantage. In order to identify complex dependencies between the elements of each input sequence, PhyloTransformer utilizes advanced modeling techniques, including a novel Fast Attention Via positive Orthogonal Random features approach (FAVOR+) from Performer, and the Masked Language Model (MLM) from Bidirectional Encoder Representations from Transformers (BERT). PhyloTransformer was trained with 1,765,297 genetic sequences retrieved from the Global Initiative for Sharing All Influenza Data (GISAID) database. Firstly, we compared the prediction accuracy of novel mutations and novel combinations using extensive baseline models; we found that PhyloTransformer outperformed every baseline method with statistical significance. Secondly, we examined predictions of mutations in each nucleotide of the receptor binding motif (RBM), and we found our predictions were precise and accurate. Thirdly, we predicted modifications of N-glycosylation sites to identify mutations associated with altered glycosylation that may be favored during viral evolution. We anticipate that PhyloTransformer may guide proactive vaccine design for effective targeting of future SARS-CoV-2 variants.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge