Learning the Physics of Particle Transport via Transformers
Paper and Code
Sep 08, 2021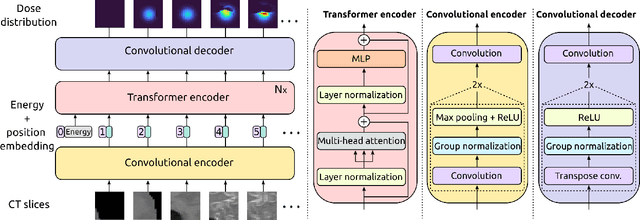
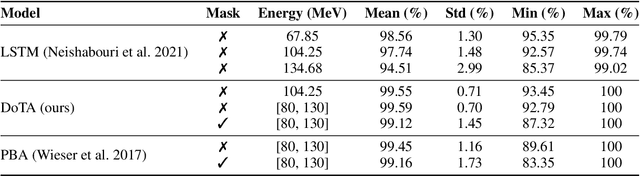
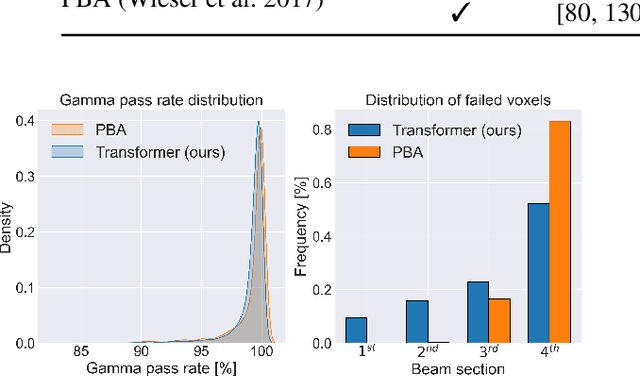
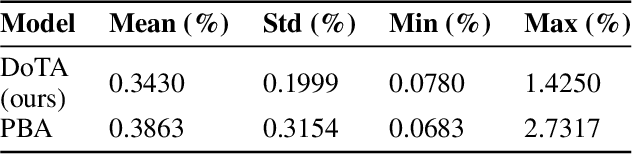
Particle physics simulations are the cornerstone of nuclear engineering applications. Among them radiotherapy (RT) is crucial for society, with 50% of cancer patients receiving radiation treatments. For the most precise targeting of tumors, next generation RT treatments aim for real-time correction during radiation delivery, necessitating particle transport algorithms that yield precise dose distributions in sub-second times even in highly heterogeneous patient geometries. This is infeasible with currently available, purely physics based simulations. In this study, we present a data-driven dose calculation algorithm predicting the dose deposited by mono-energetic proton beams for arbitrary energies and patient geometries. Our approach frames particle transport as sequence modeling, where convolutional layers extract important spatial features into tokens and the transformer self-attention mechanism routes information between such tokens in the sequence and a beam energy token. We train our network and evaluate prediction accuracy using computationally expensive but accurate Monte Carlo (MC) simulations, considered the gold standard in particle physics. Our proposed model is 33 times faster than current clinical analytic pencil beam algorithms, improving upon their accuracy in the most heterogeneous and challenging geometries. With a relative error of 0.34% and very high gamma pass rate of 99.59% (1%, 3 mm), it also greatly outperforms the only published similar data-driven proton dose algorithm, even at a finer grid resolution. Offering MC precision 400 times faster, our model could overcome a major obstacle that has so far prohibited real-time adaptive proton treatments and significantly increase cancer treatment efficacy. Its potential to model physics interactions of other particles could also boost heavy ion treatment planning procedures limited by the speed of traditional methods.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge