Contrastive Learning of Coarse-Grained Force Fields
Paper and Code
May 22, 2022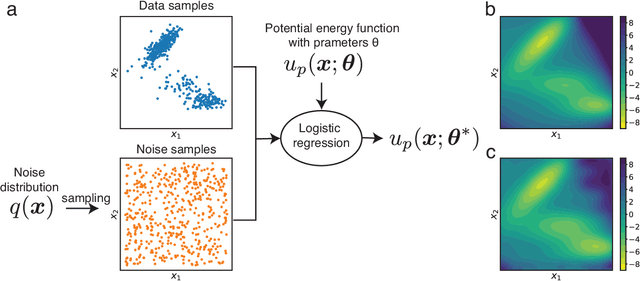
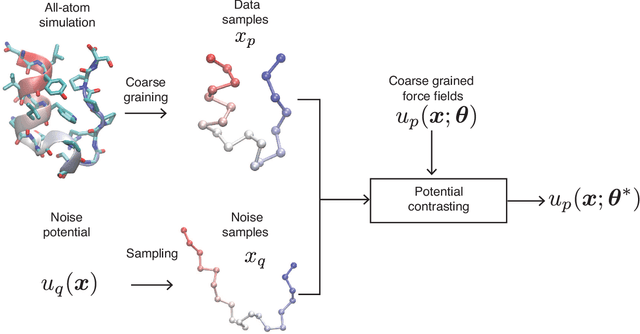
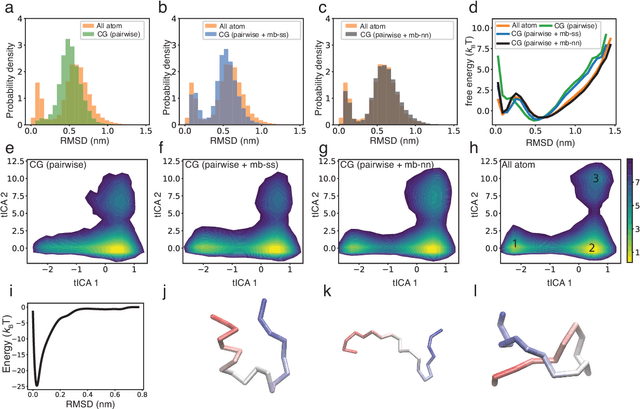
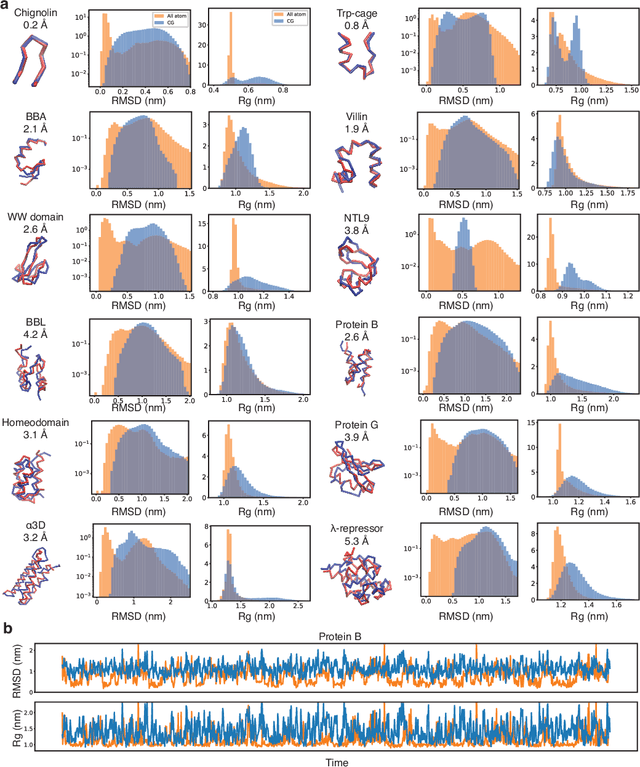
Coarse-grained models have proven helpful for simulating complex systems over long timescales to provide molecular insights into various processes. Methodologies for systematic parameterization of the underlying energy function, or force field that describes the interactions among different components of the system are of great interest for ensuring simulation accuracy. We present a new method, potential contrasting, to enable efficient learning of force fields that can accurately reproduce the conformational distribution produced with all-atom simulations. Potential contrasting generalizes the noise contrastive estimation method with umbrella sampling to better learn the complex energy landscape of molecular systems. When applied to the Trp-cage protein, we found that the technique produces force fields that thoroughly capture the thermodynamics of the folding process despite the use of only $\alpha$-Carbons in the coarse-grained model. We further showed that potential contrasting could be applied over large datasets that combine the conformational ensembles of many proteins to ensure the transferability of coarse-grained force fields. We anticipate potential contrasting to be a powerful tool for building general-purpose coarse-grained force fields.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge