Automated segmentation of microvessels in intravascular OCT images using deep learning
Paper and Code
Oct 01, 2022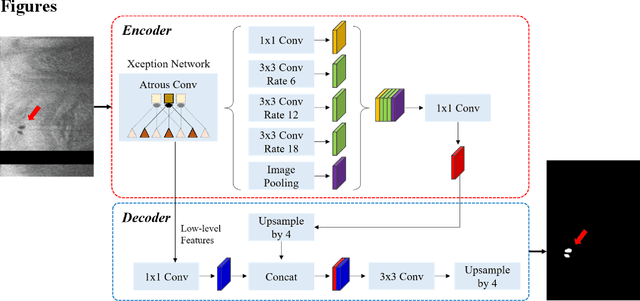
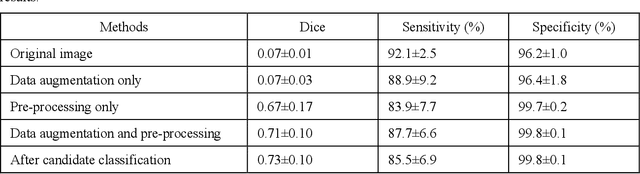

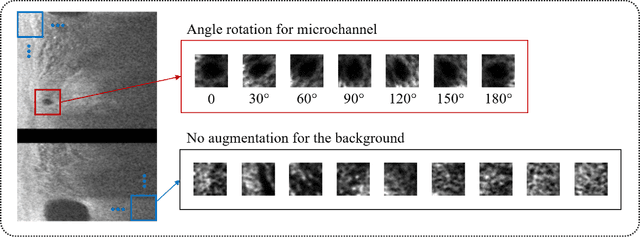
To analyze this characteristic of vulnerability, we developed an automated deep learning method for detecting microvessels in intravascular optical coherence tomography (IVOCT) images. A total of 8,403 IVOCT image frames from 85 lesions and 37 normal segments were analyzed. Manual annotation was done using a dedicated software (OCTOPUS) previously developed by our group. Data augmentation in the polar (r,{\theta}) domain was applied to raw IVOCT images to ensure that microvessels appear at all possible angles. Pre-processing methods included guidewire/shadow detection, lumen segmentation, pixel shifting, and noise reduction. DeepLab v3+ was used to segment microvessel candidates. A bounding box on each candidate was classified as either microvessel or non-microvessel using a shallow convolutional neural network. For better classification, we used data augmentation (i.e., angle rotation) on bounding boxes with a microvessel during network training. Data augmentation and pre-processing steps improved microvessel segmentation performance significantly, yielding a method with Dice of 0.71+/-0.10 and pixel-wise sensitivity/specificity of 87.7+/-6.6%/99.8+/-0.1%. The network for classifying microvessels from candidates performed exceptionally well, with sensitivity of 99.5+/-0.3%, specificity of 98.8+/-1.0%, and accuracy of 99.1+/-0.5%. The classification step eliminated the majority of residual false positives, and the Dice coefficient increased from 0.71 to 0.73. In addition, our method produced 698 image frames with microvessels present, compared to 730 from manual analysis, representing a 4.4% difference. When compared to the manual method, the automated method improved microvessel continuity, implying improved segmentation performance. The method will be useful for research purposes as well as potential future treatment planning.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge