Assigning function to protein-protein interactions: a weakly supervised BioBERT based approach using PubMed abstracts
Paper and Code
Sep 29, 2020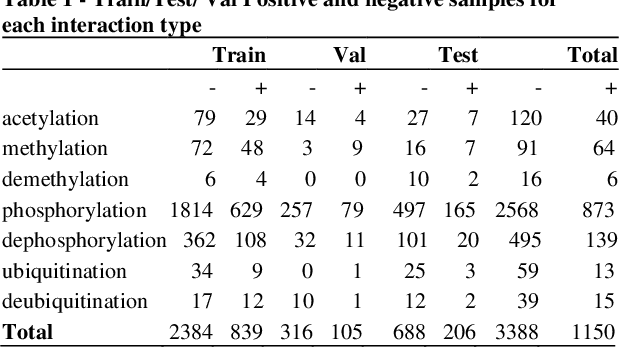

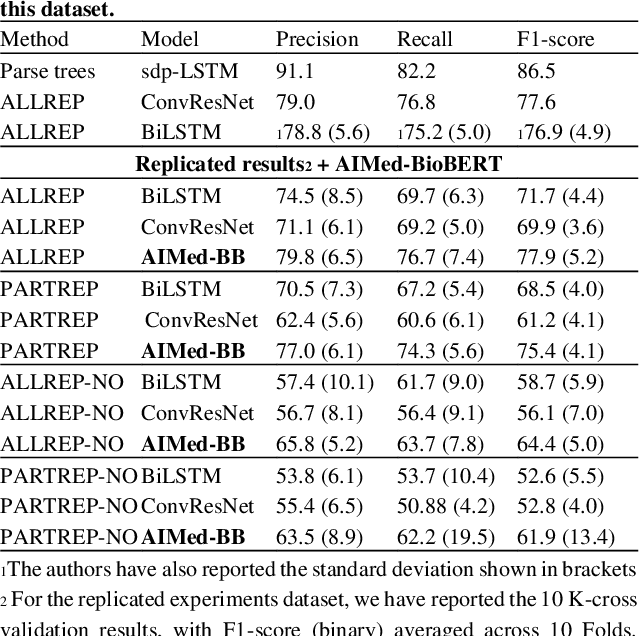
Motivation: Protein-protein interactions (PPI) are critical to the function of proteins in both normal and diseased cells, and many critical protein functions are mediated by interactions.Knowledge of the nature of these interactions is important for the construction of networks to analyse biological data. However, only a small percentage of PPIs captured in protein interaction databases have annotations of function available, e.g. only 4% of PPI are functionally annotated in the IntAct database. Here, we aim to label the function type of PPIs by extracting relationships described in PubMed abstracts. Method: We create a weakly supervised dataset from the IntAct PPI database containing interacting protein pairs with annotated function and associated abstracts from the PubMed database. We apply a state-of-the-art deep learning technique for biomedical natural language processing tasks, BioBERT, to build a model - dubbed PPI-BioBERT - for identifying the function of PPIs. In order to extract high quality PPI functions at large scale, we use an ensemble of PPI-BioBERT models to improve uncertainty estimation and apply an interaction type-specific threshold to counteract the effects of variations in the number of training samples per interaction type. Results: We scan 18 million PubMed abstracts to automatically identify 3253 new typed PPIs, including phosphorylation and acetylation interactions, with an overall precision of 46% (87% for acetylation) based on a human-reviewed sample. This work demonstrates that analysis of biomedical abstracts for PPI function extraction is a feasible approach to substantially increasing the number of interactions annotated with function captured in online databases.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge