Tran Quang Trung
BlazeNeo: Blazing fast polyp segmentation and neoplasm detection
Feb 28, 2022
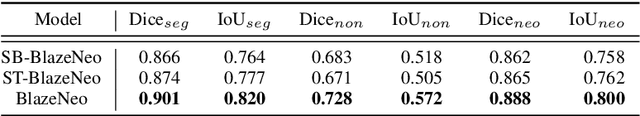
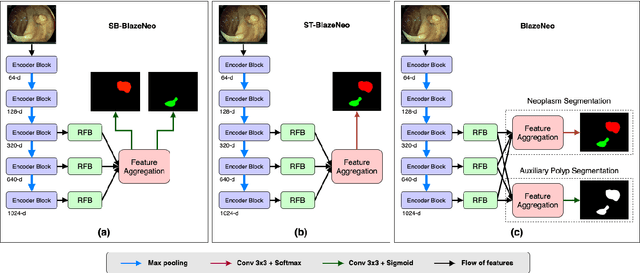
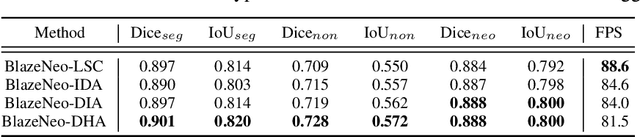
Abstract:In recent years, computer-aided automatic polyp segmentation and neoplasm detection have been an emerging topic in medical image analysis, providing valuable support to colonoscopy procedures. Attentions have been paid to improving the accuracy of polyp detection and segmentation. However, not much focus has been given to latency and throughput for performing these tasks on dedicated devices, which can be crucial for practical applications. This paper introduces a novel deep neural network architecture called BlazeNeo, for the task of polyp segmentation and neoplasm detection with an emphasis on compactness and speed while maintaining high accuracy. The model leverages the highly efficient HarDNet backbone alongside lightweight Receptive Field Blocks for computational efficiency, and an auxiliary training mechanism to take full advantage of the training data for the segmentation quality. Our experiments on a challenging dataset show that BlazeNeo achieves improvements in latency and model size while maintaining comparable accuracy against state-of-the-art methods. When deploying on the Jetson AGX Xavier edge device in INT8 precision, our BlazeNeo achieves over 155 fps while yielding the best accuracy among all compared methods.
NeoUNet: Towards accurate colon polyp segmentation and neoplasm detection
Jul 11, 2021
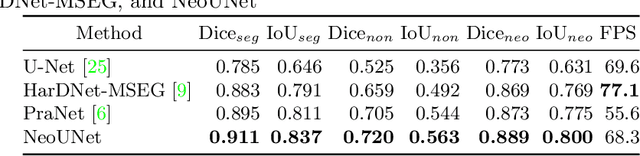


Abstract:Automatic polyp segmentation has proven to be immensely helpful for endoscopy procedures, reducing the missing rate of adenoma detection for endoscopists while increasing efficiency. However, classifying a polyp as being neoplasm or not and segmenting it at the pixel level is still a challenging task for doctors to perform in a limited time. In this work, we propose a fine-grained formulation for the polyp segmentation problem. Our formulation aims to not only segment polyp regions, but also identify those at high risk of malignancy with high accuracy. In addition, we present a UNet-based neural network architecture called NeoUNet, along with a hybrid loss function to solve this problem. Experiments show highly competitive results for NeoUNet on our benchmark dataset compared to existing polyp segmentation models.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge