Surya Seshan
Quantification of Multi-Compartment Flow with Spectral Diffusion MRI
Aug 12, 2024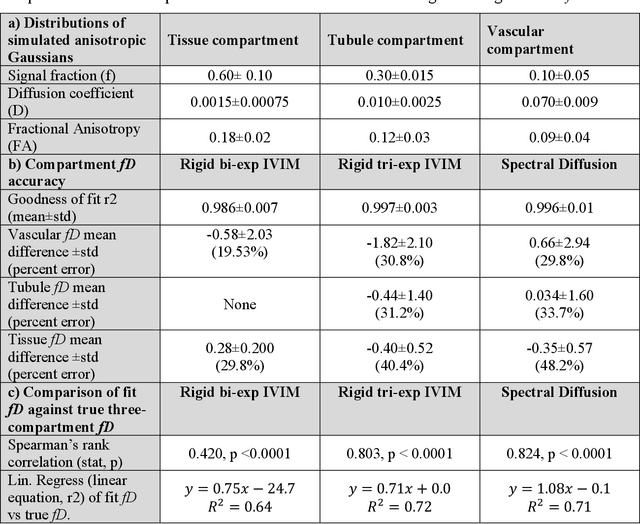

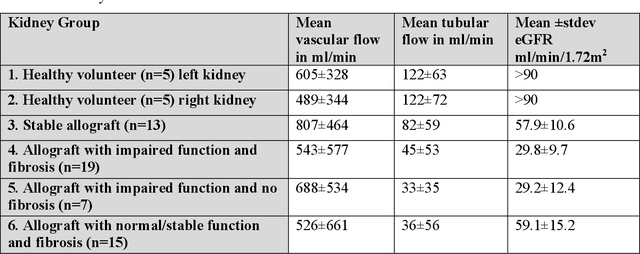

Abstract:Purpose: Estimation of multi-compartment intravoxel flow in fD in ml/100g/min with multi-b-value diffusion weighted imaging and a multi-Gaussian model in the kidneys. Theory and Methods: A multi-Gaussian model of intravoxel flow using water transport time to quantify fD is presented and simulated. Multi-compartment anisotropic DWI signal is simulated analyzed with (1) a rigid bi-exponential, (2) a rigid tri-exponential, and (3) diffusion spectrum imaging model of intravoxel incoherent motion (spectral diffusion). The application is demonstrated in a two-center study of 54 kidney allografts with 9 b-value advanced DWI that were split by function (CKD-EPI 2021 eGFR<45ml/min/1.73m2) and fibrosis (Banff 2017 interstitial fibrosis and tubular atrophy score 0-6). Results: Spectral diffusion demonstrated strong correlation to truth for simulated three-compartment anisotropic diffusion (y=1.08x+0.1, R2=0.71) and two-compartment anisotropic diffusion (y=0.91x+0.6, R2=0.74), outperforming rigid models in cases of variable compartment number. Use of a fixed regularization parameter set to {\lambda}=0.1 increased computation up to 208-fold and agreed with voxel-wise cross-validated regularization (concordance correlation coefficient=0.99). Spectral diffusion of renal allografts showed significant increase in tissue parenchyma compartment fD (f-stat=3.86, p=0.02). Tubular fD was significantly decreased in allografts with impaired function (Mann-Whitney Utest t-stat=-2.14, p=0.04). Conclusions: Quantitative multi-compartment intravoxel flow can be estimated in ml/100g/min with fD from multi-Gaussian diffusion, even with moderate anisotropy such as in kidneys. The use of spectral diffusion with a multi-Gaussian model and a fixed regularization parameter shows promise in organs such as the kidney with variable numbers of physiologic compartments.
Segmentation of diagnostic tissue compartments on whole slide images with renal thrombotic microangiopathies (TMAs)
Nov 28, 2023



Abstract:The thrombotic microangiopathies (TMAs) manifest in renal biopsy histology with a broad spectrum of acute and chronic findings. Precise diagnostic criteria for a renal biopsy diagnosis of TMA are missing. As a first step towards a machine learning- and computer vision-based analysis of wholes slide images from renal biopsies, we trained a segmentation model for the decisive diagnostic kidney tissue compartments artery, arteriole, glomerulus on a set of whole slide images from renal biopsies with TMAs and Mimickers (distinct diseases with a similar nephropathological appearance as TMA like severe benign nephrosclerosis, various vasculitides, Bevacizumab-plug glomerulopathy, arteriolar light chain deposition disease). Our segmentation model combines a U-Net-based tissue detection with a Shifted windows-transformer architecture to reach excellent segmentation results for even the most severely altered glomeruli, arterioles and arteries, even on unseen staining domains from a different nephropathology lab. With accurate automatic segmentation of the decisive renal biopsy compartments in human renal vasculopathies, we have laid the foundation for large-scale compartment-specific machine learning and computer vision analysis of renal biopsy repositories with TMAs.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge