Marina Manso Jimeno
GDCNet: Calibrationless geometric distortion correction of echo planar imaging data using deep learning
Feb 29, 2024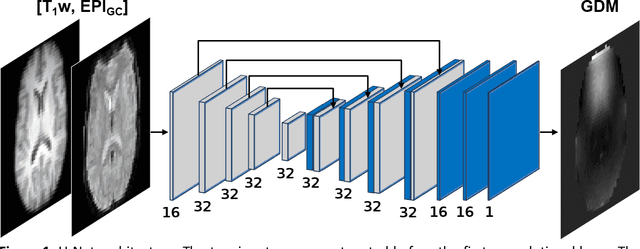


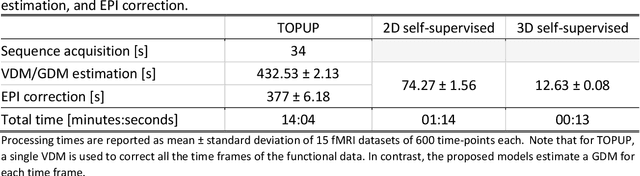
Abstract:Functional magnetic resonance imaging techniques benefit from echo-planar imaging's fast image acquisition but are susceptible to inhomogeneities in the main magnetic field, resulting in geometric distortion and signal loss artifacts in the images. Traditional methods leverage a field map or voxel displacement map for distortion correction. However, voxel displacement map estimation requires additional sequence acquisitions, and the accuracy of the estimation influences correction performance. This work implements a novel approach called GDCNet, which estimates a geometric distortion map by non-linear registration to T1-weighted anatomical images and applies it for distortion correction. GDCNet demonstrated fast distortion correction of functional images in retrospectively and prospectively acquired datasets. Among the compared models, the 2D self-supervised configuration resulted in a statistically significant improvement to normalized mutual information between distortion-corrected functional and T1-weighted images compared to the benchmark methods FUGUE and TOPUP. Furthermore, GDCNet models achieved processing speeds 14 times faster than TOPUP in the prospective dataset.
Automated detection of motion artifacts in brain MR images using deep learning and explainable artificial intelligence
Feb 13, 2024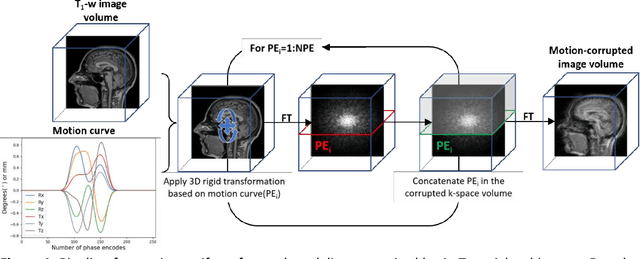


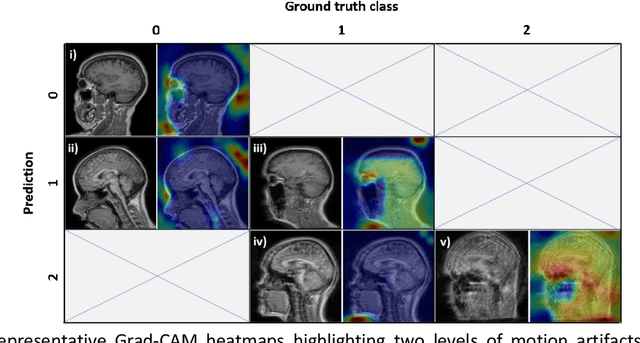
Abstract:Quality assessment, including inspecting the images for artifacts, is a critical step during MRI data acquisition to ensure data quality and downstream analysis or interpretation success. This study demonstrates a deep learning model to detect rigid motion in T1-weighted brain images. We leveraged a 2D CNN for three-class classification and tested it on publicly available retrospective and prospective datasets. Grad-CAM heatmaps enabled the identification of failure modes and provided an interpretation of the model's results. The model achieved average precision and recall metrics of 85% and 80% on six motion-simulated retrospective datasets. Additionally, the model's classifications on the prospective dataset showed a strong inverse correlation (-0.84) compared to average edge strength, an image quality metric indicative of motion. This model is part of the ArtifactID tool, aimed at inline automatic detection of Gibbs ringing, wrap-around, and motion artifacts. This tool automates part of the time-consuming QA process and augments expertise on-site, particularly relevant in low-resource settings where local MR knowledge is scarce.
Developing and deploying deep learning models in brain MRI: a review
Jan 03, 2023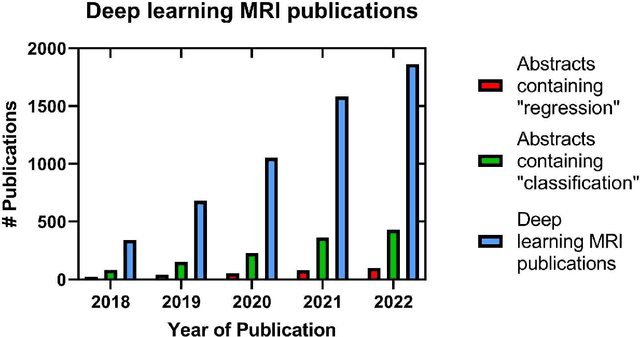



Abstract:Magnetic Resonance Imaging (MRI) of the brain has benefited from deep learning (DL) to alleviate the burden on radiologists and MR technologists, and improve throughput. The easy accessibility of DL tools have resulted in the rapid increase of DL models and subsequent peer-reviewed publications. However, the rate of deployment in clinical settings is low. Therefore, this review attempts to bring together the ideas from data collection to deployment into the clinic building on the guidelines and principles that accreditation agencies have espoused. We introduce the need for and the role of DL to deliver accessible MRI. This is followed by a brief review of DL examples in the context of neuropathologies. Based on these studies and others, we collate the prerequisites to develop and deploy DL models for brain MRI. We then delve into the guiding principles to practice good machine learning practices in the context of neuroimaging with a focus on explainability. A checklist based on the FDA's good machine learning practices is provided as a summary of these guidelines. Finally, we review the current challenges and future opportunities in DL for brain MRI.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge