Jochen G. Hirsch
Depth to Anatomy: Learning Internal Organ Locations from Surface Depth Images
Jan 26, 2026Abstract:Automated patient positioning plays an important role in optimizing scanning procedure and improving patient throughput. Leveraging depth information captured by RGB-D cameras presents a promising approach for estimating internal organ positions, thereby enabling more accurate and efficient positioning. In this work, we propose a learning-based framework that directly predicts the 3D locations and shapes of multiple internal organs from single 2D depth images of the body surface. Utilizing a large-scale dataset of full-body MRI scans, we synthesize depth images paired with corresponding anatomical segmentations to train a unified convolutional neural network architecture. Our method accurately localizes a diverse set of anatomical structures, including bones and soft tissues, without requiring explicit surface reconstruction. Experimental results demonstrate the potential of integrating depth sensors into radiology workflows to streamline scanning procedures and enhance patient experience through automated patient positioning.
Internal Organ Localization Using Depth Images
Mar 30, 2025Abstract:Automated patient positioning is a crucial step in streamlining MRI workflows and enhancing patient throughput. RGB-D camera-based systems offer a promising approach to automate this process by leveraging depth information to estimate internal organ positions. This paper investigates the feasibility of a learning-based framework to infer approximate internal organ positions from the body surface. Our approach utilizes a large-scale dataset of MRI scans to train a deep learning model capable of accurately predicting organ positions and shapes from depth images alone. We demonstrate the effectiveness of our method in localization of multiple internal organs, including bones and soft tissues. Our findings suggest that RGB-D camera-based systems integrated into MRI workflows have the potential to streamline scanning procedures and improve patient experience by enabling accurate and automated patient positioning.
Self-supervised Learning of Dense Hierarchical Representations for Medical Image Segmentation
Jan 12, 2024
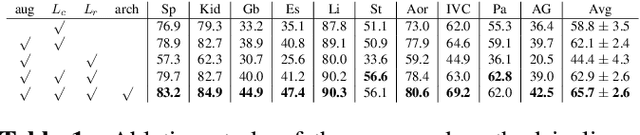
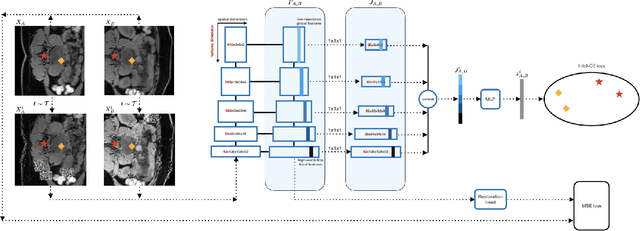
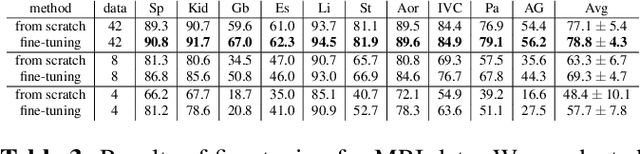
Abstract:This paper demonstrates a self-supervised framework for learning voxel-wise coarse-to-fine representations tailored for dense downstream tasks. Our approach stems from the observation that existing methods for hierarchical representation learning tend to prioritize global features over local features due to inherent architectural bias. To address this challenge, we devise a training strategy that balances the contributions of features from multiple scales, ensuring that the learned representations capture both coarse and fine-grained details. Our strategy incorporates 3-fold improvements: (1) local data augmentations, (2) a hierarchically balanced architecture, and (3) a hybrid contrastive-restorative loss function. We evaluate our method on CT and MRI data and demonstrate that our new approach particularly beneficial for fine-tuning with limited annotated data and consistently outperforms the baseline counterpart in linear evaluation settings.
An Uncertainty-Aware, Shareable and Transparent Neural Network Architecture for Brain-Age Modeling
Jul 16, 2021
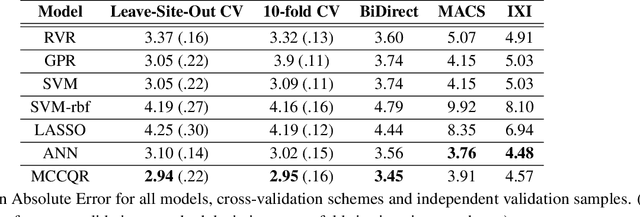
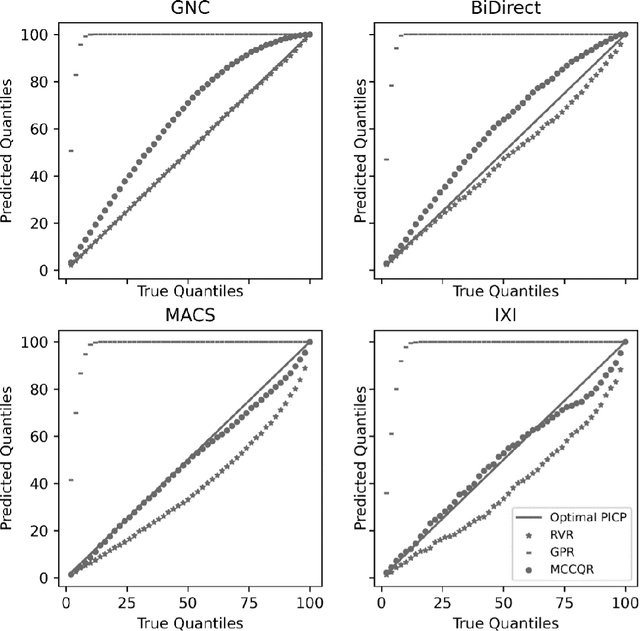
Abstract:The deviation between chronological age and age predicted from neuroimaging data has been identified as a sensitive risk-marker of cross-disorder brain changes, growing into a cornerstone of biological age-research. However, Machine Learning models underlying the field do not consider uncertainty, thereby confounding results with training data density and variability. Also, existing models are commonly based on homogeneous training sets, often not independently validated, and cannot be shared due to data protection issues. Here, we introduce an uncertainty-aware, shareable, and transparent Monte-Carlo Dropout Composite-Quantile-Regression (MCCQR) Neural Network trained on N=10,691 datasets from the German National Cohort. The MCCQR model provides robust, distribution-free uncertainty quantification in high-dimensional neuroimaging data, achieving lower error rates compared to existing models across ten recruitment centers and in three independent validation samples (N=4,004). In two examples, we demonstrate that it prevents spurious associations and increases power to detect accelerated brain-aging. We make the pre-trained model publicly available.
Predicting brain-age from raw T 1 -weighted Magnetic Resonance Imaging data using 3D Convolutional Neural Networks
Mar 22, 2021



Abstract:Age prediction based on Magnetic Resonance Imaging (MRI) data of the brain is a biomarker to quantify the progress of brain diseases and aging. Current approaches rely on preparing the data with multiple preprocessing steps, such as registering voxels to a standardized brain atlas, which yields a significant computational overhead, hampers widespread usage and results in the predicted brain-age to be sensitive to preprocessing parameters. Here we describe a 3D Convolutional Neural Network (CNN) based on the ResNet architecture being trained on raw, non-registered T$_ 1$-weighted MRI data of N=10,691 samples from the German National Cohort and additionally applied and validated in N=2,173 samples from three independent studies using transfer learning. For comparison, state-of-the-art models using preprocessed neuroimaging data are trained and validated on the same samples. The 3D CNN using raw neuroimaging data predicts age with a mean average deviation of 2.84 years, outperforming the state-of-the-art brain-age models using preprocessed data. Since our approach is invariant to preprocessing software and parameter choices, it enables faster, more robust and more accurate brain-age modeling.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge