Jacob Bergsland
Multimodal Deep Learning for Personalized Renal Cell Carcinoma Prognosis: Integrating CT Imaging and Clinical Data
Jul 07, 2023



Abstract:Renal cell carcinoma represents a significant global health challenge with a low survival rate. This research aimed to devise a comprehensive deep-learning model capable of predicting survival probabilities in patients with renal cell carcinoma by integrating CT imaging and clinical data and addressing the limitations observed in prior studies. The aim is to facilitate the identification of patients requiring urgent treatment. The proposed framework comprises three modules: a 3D image feature extractor, clinical variable selection, and survival prediction. The feature extractor module, based on the 3D CNN architecture, predicts the ISUP grade of renal cell carcinoma tumors linked to mortality rates from CT images. A selection of clinical variables is systematically chosen using the Spearman score and random forest importance score as criteria. A deep learning-based network, trained with discrete LogisticHazard-based loss, performs the survival prediction. Nine distinct experiments are performed, with varying numbers of clinical variables determined by different thresholds of the Spearman and importance scores. Our findings demonstrate that the proposed strategy surpasses the current literature on renal cancer prognosis based on CT scans and clinical factors. The best-performing experiment yielded a concordance index of 0.84 and an area under the curve value of 0.8 on the test cohort, which suggests strong predictive power. The multimodal deep-learning approach developed in this study shows promising results in estimating survival probabilities for renal cell carcinoma patients using CT imaging and clinical data. This may have potential implications in identifying patients who require urgent treatment, potentially improving patient outcomes. The code created for this project is available for the public on: \href{https://github.com/Balasingham-AI-Group/Survival_CTplusClinical}{GitHub}
Accurate Real-time Polyp Detection in Videos from Concatenation of Latent Features Extracted from Consecutive Frames
Mar 10, 2023



Abstract:An efficient deep learning model that can be implemented in real-time for polyp detection is crucial to reducing polyp miss-rate during screening procedures. Convolutional neural networks (CNNs) are vulnerable to small changes in the input image. A CNN-based model may miss the same polyp appearing in a series of consecutive frames and produce unsubtle detection output due to changes in camera pose, lighting condition, light reflection, etc. In this study, we attempt to tackle this problem by integrating temporal information among neighboring frames. We propose an efficient feature concatenation method for a CNN-based encoder-decoder model without adding complexity to the model. The proposed method incorporates extracted feature maps of previous frames to detect polyps in the current frame. The experimental results demonstrate that the proposed method of feature concatenation improves the overall performance of automatic polyp detection in videos. The following results are obtained on a public video dataset: sensitivity 90.94\%, precision 90.53\%, and specificity 92.46%
The End-to-End Molecular Communication Model of Extracellular Vesicle-based Drug Delivery
Jul 05, 2022



Abstract:A closer look at nature has recently brought more interest in exploring and utilizing intra-body communication networks composed of cells as intrinsic, perfectly biocompatible infrastructures to deliver therapeutics. Naturally occurring cell-to-cell communication systems are being manipulated to release, navigate, and take up soluble cell-derived messengers that are either therapeutic by nature or carry therapeutic molecular cargo in their structures. One example of such structures is extracellular vesicles (EVs) which have been recently proven to have favorable pharmacokinetic properties, opening new avenues for developing the next generation biotherapeutics. In this paper, we study theoretical aspects of the EV transfer within heart tissue as a case study by utilizing an information and communication technology-like approach in analyzing molecular communication systems. Our modeling implies the abstraction of the EV releasing cells as transmitters, the extracellular matrix as the channel, and the EV receiving cells as receivers. Our results, derived from the developed analytical models, indicate that the release can be modulated using external forces such as electrical signals, and the transfer and reception can be affected by the extracellular matrix and plasma membrane properties, respectively. The results can predict the EV biodistributions and contribute to avoiding unplanned administration, often resulting in side- and adverse effects.
Polyp Detection and Segmentation using Mask R-CNN: Does a Deeper Feature Extractor CNN Always Perform Better?
Jul 22, 2019
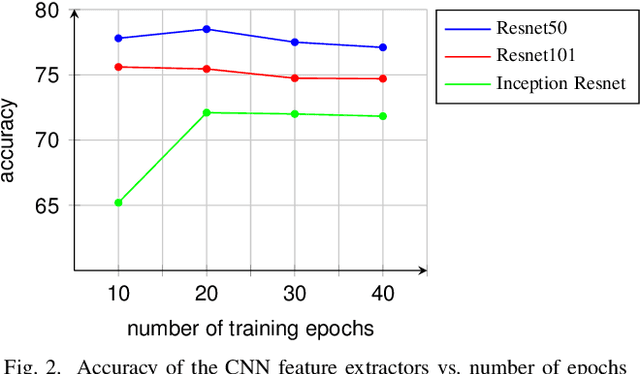


Abstract:Automatic polyp detection and segmentation are highly desirable for colon screening due to polyp miss rate by physicians during colonoscopy, which is about 25%. However, this computerization is still an unsolved problem due to various polyp-like structures in the colon and high interclass polyp variations in terms of size, color, shape, and texture. In this paper, we adapt Mask R-CNN and evaluate its performance with different modern convolutional neural networks (CNN) as its feature extractor for polyp detection and segmentation. We investigate the performance improvement of each feature extractor by adding extra polyp images to the training dataset to answer whether we need deeper and more complex CNNs or better dataset for training in automatic polyp detection and segmentation. Finally, we propose an ensemble method for further performance improvement. We evaluate the performance on the 2015 MICCAI polyp detection dataset. The best results achieved are 72.59% recall, 80% precision, 70.42% dice, and 61.24% Jaccard. The model achieved state-of-the-art segmentation performance.
* 6
Automatic Colon Polyp Detection using Region based Deep CNN and Post Learning Approaches
Jun 27, 2019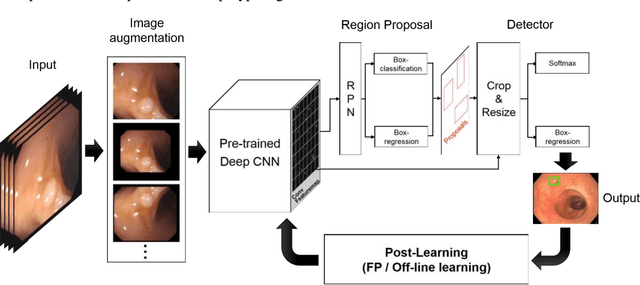



Abstract:Automatic detection of colonic polyps is still an unsolved problem due to the large variation of polyps in terms of shape, texture, size, and color, and the existence of various polyp-like mimics during colonoscopy. In this study, we apply a recent region based convolutional neural network (CNN) approach for the automatic detection of polyps in images and videos obtained from colonoscopy examinations. We use a deep-CNN model (Inception Resnet) as a transfer learning scheme in the detection system. To overcome the polyp detection obstacles and the small number of polyp images, we examine image augmentation strategies for training deep networks. We further propose two efficient post-learning methods such as, automatic false positive learning and off-line learning, both of which can be incorporated with the region based detection system for reliable polyp detection. Using the large size of colonoscopy databases, experimental results demonstrate that the suggested detection systems show better performance compared to other systems in the literature. Furthermore, we show improved detection performance using the proposed post-learning schemes for colonoscopy videos.
* 9 pages
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge