Farshad Moradi
Score-based Generative Diffusion Models to Synthesize Full-dose FDG Brain PET from MRI in Epilepsy Patients
Jun 12, 2025Abstract:Fluorodeoxyglucose (FDG) PET to evaluate patients with epilepsy is one of the most common applications for simultaneous PET/MRI, given the need to image both brain structure and metabolism, but is suboptimal due to the radiation dose in this young population. Little work has been done synthesizing diagnostic quality PET images from MRI data or MRI data with ultralow-dose PET using advanced generative AI methods, such as diffusion models, with attention to clinical evaluations tailored for the epilepsy population. Here we compared the performance of diffusion- and non-diffusion-based deep learning models for the MRI-to-PET image translation task for epilepsy imaging using simultaneous PET/MRI in 52 subjects (40 train/2 validate/10 hold-out test). We tested three different models: 2 score-based generative diffusion models (SGM-Karras Diffusion [SGM-KD] and SGM-variance preserving [SGM-VP]) and a Transformer-Unet. We report results on standard image processing metrics as well as clinically relevant metrics, including congruency measures (Congruence Index and Congruency Mean Absolute Error) that assess hemispheric metabolic asymmetry, which is a key part of the clinical analysis of these images. The SGM-KD produced the best qualitative and quantitative results when synthesizing PET purely from T1w and T2 FLAIR images with the least mean absolute error in whole-brain specific uptake value ratio (SUVR) and highest intraclass correlation coefficient. When 1% low-dose PET images are included in the inputs, all models improve significantly and are interchangeable for quantitative performance and visual quality. In summary, SGMs hold great potential for pure MRI-to-PET translation, while all 3 model types can synthesize full-dose FDG-PET accurately using MRI and ultralow-dose PET.
Closed-loop control of seizure activity via real-time seizure forecasting by reservoir neuromorphic computing
May 04, 2025Abstract:Closed-loop brain stimulation holds potential as personalized treatment for drug-resistant epilepsy (DRE) but still suffers from limitations that result in highly variable efficacy. First, stimulation is typically delivered upon detection of the seizure to abort rather than prevent it; second, the stimulation parameters are established by trial and error, requiring lengthy rounds of fine-tuning, which delay steady-state therapeutic efficacy. Here, we address these limitations by leveraging the potential of neuromorphic computing. We present a system capable of driving personalized free-run stimulations based on seizure forecasting, wherein each forecast triggers an electrical pulse rather than an arbitrarily predefined fixed-frequency stimulus train. We validate the system against hippocampal spheroids coupled to 3D microelectrode array as a simplified testbed, showing that it can achieve seizure reduction >97% while primarily using instantaneous stimulation frequencies within 20 Hz, well below what typically used in clinical settings. Our work demonstrates the potential of neuromorphic systems as a next-generation neuromodulation strategy for personalized DRE treatment.
NET-TEN: a silicon neuromorphic network for low-latency detection of seizures in local field potentials
Oct 19, 2022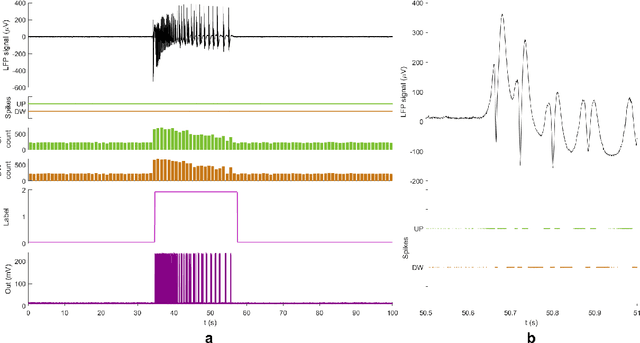
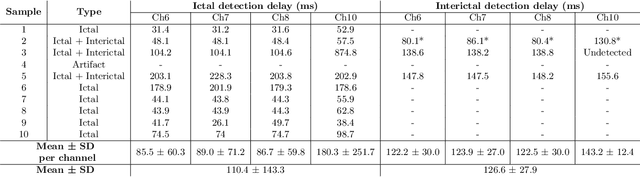
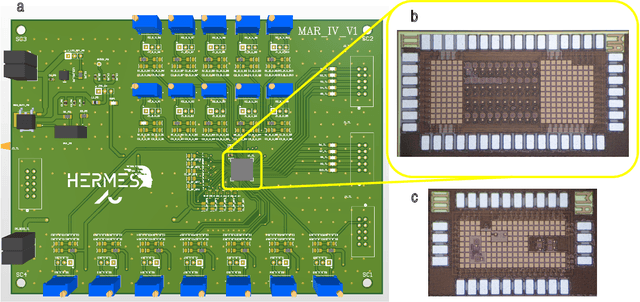
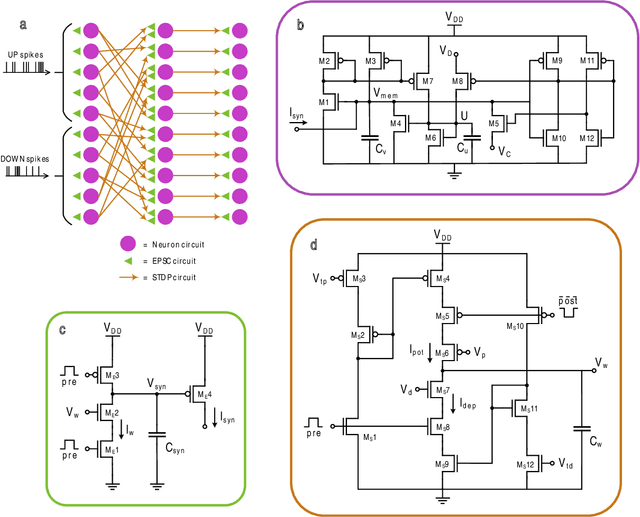
Abstract:Therapeutic intervention in neurological disorders still relies heavily on pharmacological solutions, while the treatment of patients with drug resistance remains an open challenge. This is particularly true for patients with epilepsy, 30% of whom are refractory to medications. Implantable devices for chronic recording and electrical modulation of brain activity have proved a viable alternative in such cases. To operate, the device should detect the relevant electrographic biomarkers from Local Field Potentials (LFPs) and determine the right time for stimulation. To enable timely interventions, the ideal device should attain biomarker detection with low latency while operating under low power consumption to prolong the battery life. Neuromorphic networks have progressively gained reputation as low-latency low-power computing systems, which makes them a promising candidate as processing core of next-generation implantable neural interfaces. Here we introduce a fully-analog neuromorphic device implemented in CMOS technology for analyzing LFP signals in an in vitro model of acute ictogenesis. We show that the system can detect ictal and interictal events with ms-latency and with high precision, consuming on average 3.50 nW during the task. Our work paves the way to a new generation of brain implantable devices for personalized closed-loop stimulation for epilepsy treatment.
In the realm of hybrid Brain: Human Brain and AI
Oct 07, 2022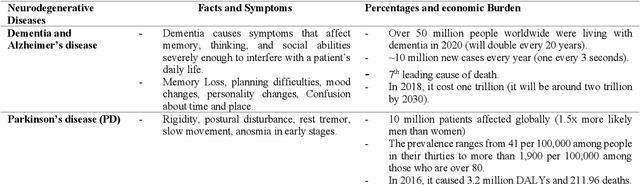
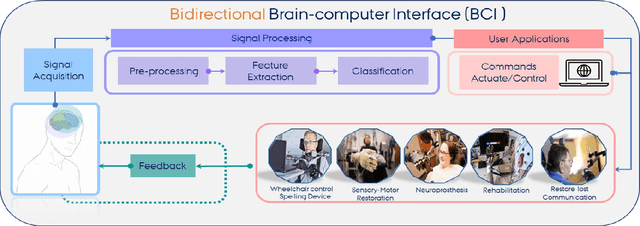
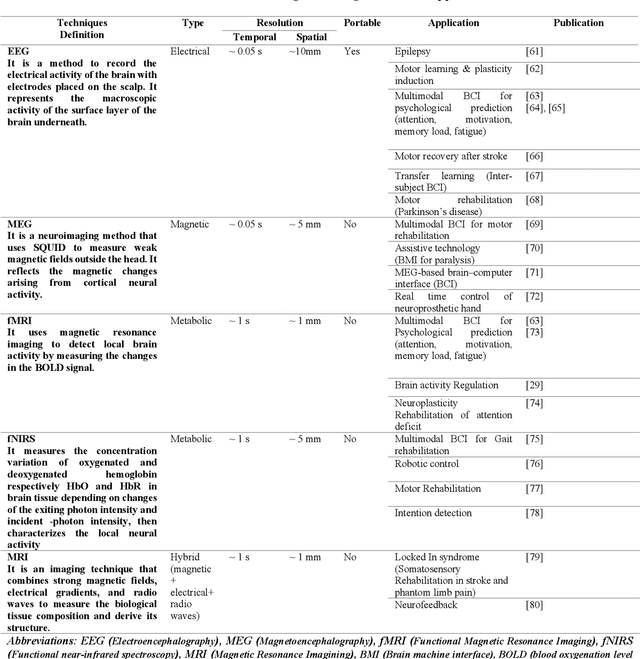
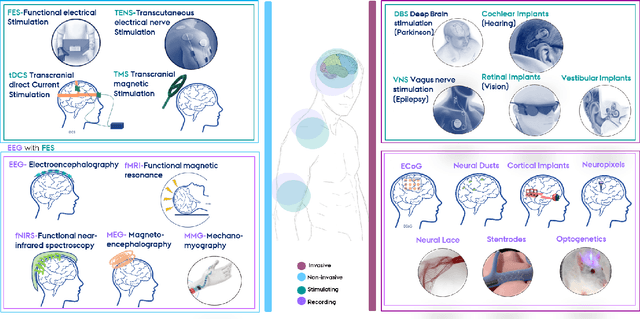
Abstract:With the recent developments in neuroscience and engineering, it is now possible to record brain signals and decode them. Also, a growing number of stimulation methods have emerged to modulate and influence brain activity. Current brain-computer interface (BCI) technology is mainly on therapeutic outcomes, it already demonstrated its efficiency as assistive and rehabilitative technology for patients with severe motor impairments. Recently, artificial intelligence (AI) and machine learning (ML) technologies have been used to decode brain signals. Beyond this progress, combining AI with advanced BCIs in the form of implantable neurotechnologies grants new possibilities for the diagnosis, prediction, and treatment of neurological and psychiatric disorders. In this context, we envision the development of closed loop, intelligent, low-power, and miniaturized neural interfaces that will use brain inspired AI techniques with neuromorphic hardware to process the data from the brain. This will be referred to as Brain Inspired Brain Computer Interfaces (BI-BCIs). Such neural interfaces would offer access to deeper brain regions and better understanding for brain's functions and working mechanism, which improves BCIs operative stability and system's efficiency. On one hand, brain inspired AI algorithms represented by spiking neural networks (SNNs) would be used to interpret the multimodal neural signals in the BCI system. On the other hand, due to the ability of SNNs to capture rich dynamics of biological neurons and to represent and integrate different information dimensions such as time, frequency, and phase, it would be used to model and encode complex information processing in the brain and to provide feedback to the users. This paper provides an overview of the different methods to interface with the brain, presents future applications and discusses the merger of AI and BCIs.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge